Stable Cell Line Construction for Protein Expression
Background
Introduction
Stable cell lines, such as CHO and HEK cells, are pivotal in accelerating biopharmaceutical innovation, offering reliable platforms for recombinant protein expression, biologics production, and high-throughput drug screening. These genetically engineered cell lines ensure consistent, scalable synthesis of therapeutic proteins and antibodies, driving breakthroughs in drug discovery and therapeutic development. At Profacgen, we combine cutting-edge gene integration, clonal selection, and validation technologies to deliver high-yield, regulatory-compliant cell lines tailored for your projects-from monoclonal antibody engineering to toxicology studies.
Contact Us Today to Streamline Your Biologics Pipeline!
Why Choose Stable Cell Lines?
| Consistent and Reproducible Protein Expression Stable cell lines ensure uniform production of recombinant proteins, minimizing batch-to-batch variability—a critical requirement for drug development and quality control. By integrating target genes into host genomes (e.g., CHO or HEK cells), these cell lines maintain consistent protein expression over extended periods, supporting reliable data generation in therapeutic antibody development, enzyme studies, and biomarker research. |
| Long-Term Viability and Scalable Production Engineered for scalable production, stable cell lines offer unmatched durability in continuous culture, enabling large-scale biologics manufacturing without compromising yield or stability. Their adaptability to bioreactor systems streamlines transition from research to commercialization, making them ideal for vaccines, monoclonal antibodies, and biosimilars requiring high-volume output. |
| Cost-Effective Bioproduction for Industrial Applications Stable cell line technology reduces long-term operational costs by eliminating the need for transient transfection and repetitive gene delivery. This cost-effective bioproduction approach optimizes resource utilization, lowers per-unit protein costs, and accelerates timelines for GMP-compliant therapeutics, positioning it as a cornerstone for sustainable, industrial-scale biologics manufacturing. |
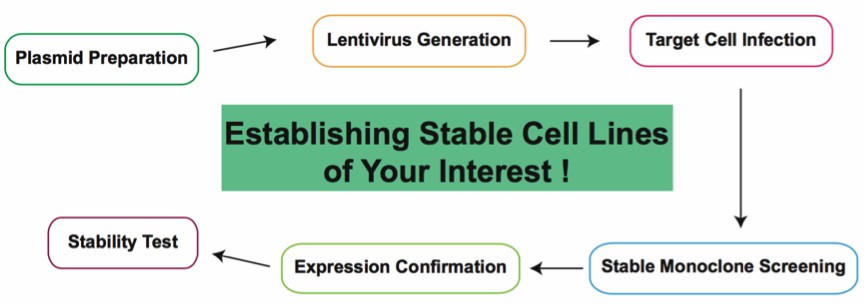 Fig1. Workflow for Establishing Stable Cell Lines.
Fig1. Workflow for Establishing Stable Cell Lines.
Applications and Industries
| Therapeutic Development |
|
| Drug Discovery & Screening |
|
| Diagnostic & Research Tools |
|
| Industrial Bioproduction |
|
| Functional Genomics |
|
Service Procedure
Sample Submission Requirements
- Protein Sequence Information (Required): The amino acid sequence of the target protein must be provided.
- Selection Marker (Provided by Profacgen): We will provide the selection marker to be used post-transfection.
- Other Requirments (Optional):
- Expression Vector: If available, details or the source of the expression vector can be submitted.
- Host Cell Information: Specify the type of host cells if you have a preference; otherwise, we can recommend suitable options.
- Growth Conditions: Provide any specific growth conditions if known; otherwise, our standard conditions will be applied.
- Quality Control Data: Submit any available QC data on the provided samples to expedite the process.
- Special Instructions: Include any special handling or processing instructions that are relevant to your project.
Our Stable Cell Line Development Services
At Profacgen, we specialize in delivering end-to-end solutions for constructing high-performance stable cell lines tailored to your research or bioproduction needs. Leveraging cutting-edge technologies and rigorous quality control protocols, our services ensure reliable, scalable, and reproducible outcomes for diverse applications.
1. Precision Gene Delivery & Targeted Integration
Achieve optimal gene expression with our advanced gene delivery systems. Utilizing high-efficiency lentiviral vectors, we ensure:
- >90% transfection efficiency for robust gene insertion.
- Site-specific integration to minimize positional effects.
- Genetic stability validation via Southern blot and NGS analysis.
Ideal for long-term protein production studies or therapeutic molecule development.
2. High-Yield Monoclone Screening & Selection
Isolate genetically homogeneous high-yield monoclones through our multi-stage screening pipeline:
- Automated limited dilution cloning for single-cell certainty.
- Dual antibiotic selection (e.g., puromycin/neomycin) for stringent pressure.
- Flow cytometry screening combined with ELISA to identify top producers.
Guaranteed >95% monoclonality with full documentation of clone lineage.
3. Customizable Protein Expression Systems
Design cell lines that match your exact specifications:
- Tet-ON inducible cell lines for temporal control of expression.
- Complex molecule production:
- Bispecific antibodies & biosimilars
- Tagged fusion proteins (His, FLAG, Fc)
- Cytokines (IL-2, IFN-γ) & growth factors (VEGF, FGF)
- Species options: CHO, HEK293, or custom host cells.
4. Rigorous Quality Assurance & Stability Testing
Every cell line undergoes systematic validation. Adherence to international quality frameworks and manufacturing-aligned protocols, ensuring safety and reproducibility.
| Test Category | Key Methods |
| Identity | STR profiling, Isozyme analysis |
| Purity | Mycoplasma PCR, endotoxin assays |
| Function | qRT-PCR, Western blot, ICC/IF |
| Stability | 60+ day passaging with productivity tracking |
Why Choose Profacgen?
- Expert Cell Line Development
15+ years of specialized expertise in recombinant protein systems, supported by patented workflows and PhD-led innovation. - Advanced Biotechnology Facilities
Lentiviral, AI-driven platforms, and ISO-certified labs enable precision engineering for high-yield monoclones. - One-Stop Service Efficiency
Seamless integration from gene delivery to clone validation-GMP-ready cell lines in 10-14 weeks. - Rigorous Quality Assurance
Stability testing (60+ day passaging) and flow cytometry screening ensure batch-to-batch consistency. - IP-Protected Solutions
Confidentiality agreements and patent-compliant processes safeguard your intellectual property.
Case Study
* NOTE: We prioritize confidentiality in our services to safeguard technology and intellectual property for enhanced future value and protection. The following case study has been shared with the client's consent.
Goal
The project involves the generation of a stable cell line, starting from transient expression in ExpiCHO cells, followed by the construction of a CHO stable cell line. The goal is to obtain a stable cell line that can efficiently produce the desired protein.
Results
1. Protein Sequence: The theoretical molecular weight of the target protein was determined to be 37.7 kDa.
2. Target Protein Expression and Purification: The constructed plasmid was transfected into ExpiCHO cells, cultured for 6 days, and the supernatant was purified using a Protein A affinity column. Western Blot analysis confirmed successful protein expression and purification.
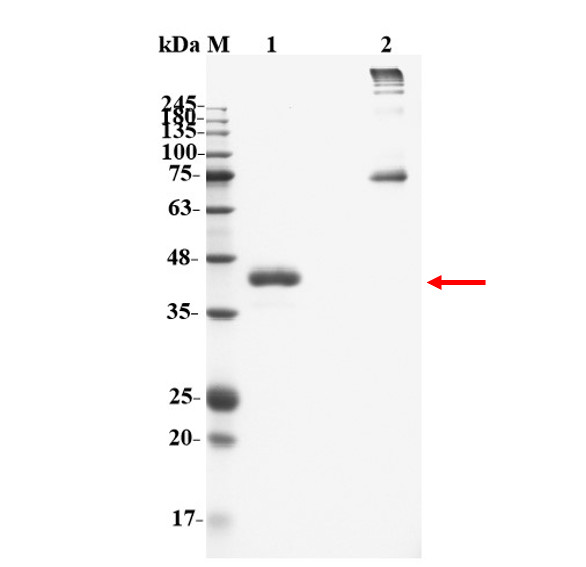 Fig2. WB analysis of protein purification.
Fig2. WB analysis of protein purification.
Lane 1: Reducing (2μg);
Lane 2: Non-reducing (2μg).
3. Minipool Cell Screening: A total of 102 clones were screened using the Dot Blot method, and 55 minipools were selected for further analysis. Western Blot analysis identified five high-expressing minipools (SC11, SD03, SD12, SD39, and SD46).
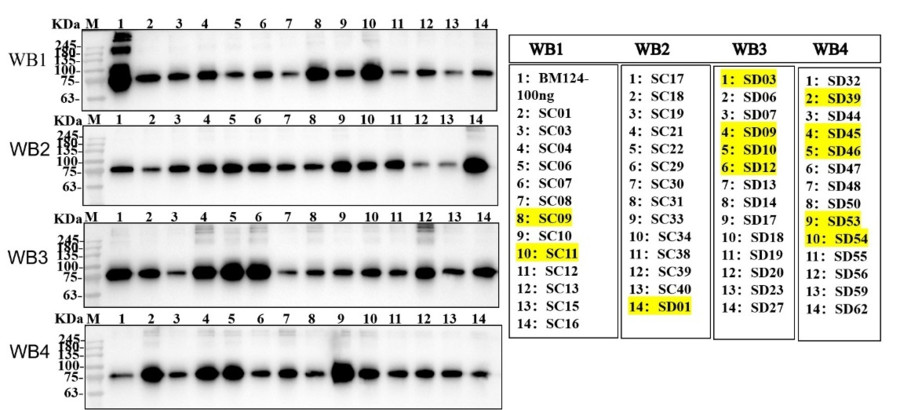 Fig3. WB analysis of 55 minipools.
Fig3. WB analysis of 55 minipools.
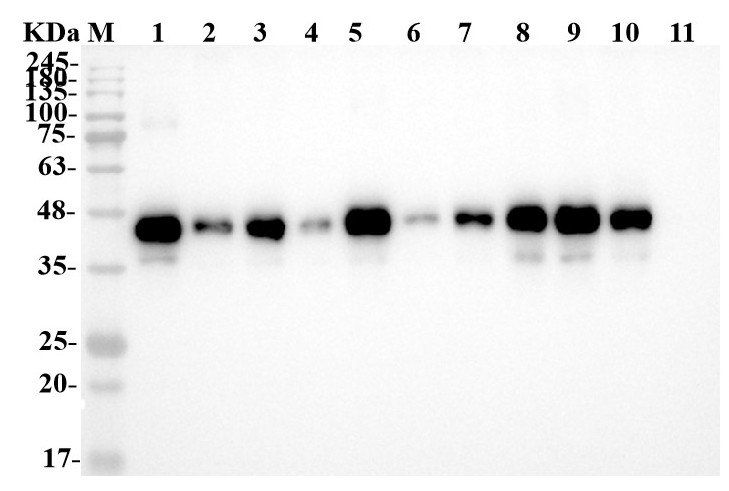 Fig4. WB analysis of 10 minipools.
Fig4. WB analysis of 10 minipools.
Lane 1: Positive control
Lane 2-11: SC09, SC11, SD01, SD03, SD09, SD10, SD12, SD39, SD46, SD54.
4. Shake Flask Expression: The selected minipools were cultured in shake flasks, and their growth and protein expression levels were monitored. SDS-PAGE analysis showed good expression levels.
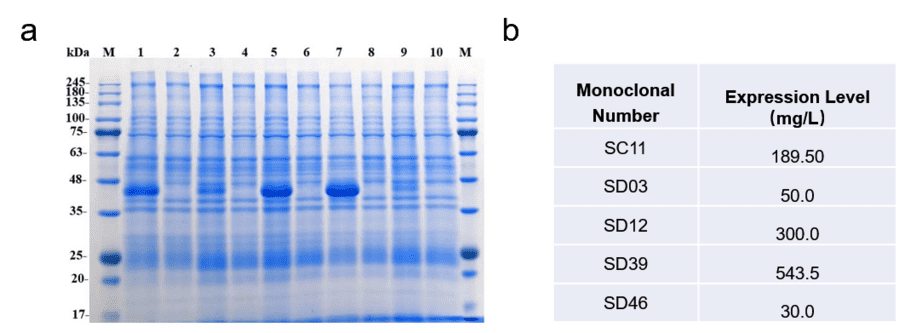
Fig5. SDS-PAGE analysis and expression level of 5 minipools.
5. Monoclonal Screening: Further screening of monoclonal cells identified eight high-expressing clones (SC11-16, SC11-18, SC11-19, SC11-20, SC11-21, SD03-23, SD46-39, and SD46-41). HPLC analysis indicated that the purity of the purified proteins from these clones was above 98%.
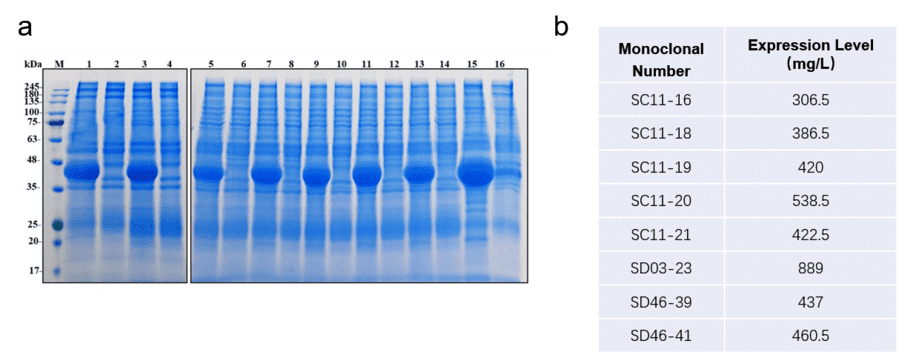
Fig6. SDS-PAGE analysis and expression level of 8 minipools.
6. Stable Cell Line Optimization: The optimized stable cell line (SD03-23) achieved a protein expression level of 889 mg/ml. After optimization, the protein yield was measured at 1.00 g/L, 1.45 g/L, and 1.62 g/L for three different feed batch groups.
Conclusions and Discussions
- The project successfully constructed recombinant plasmids and achieved high-level protein expression in transiently transfected ExpiCHO cells.
- Eight high-expressing CHO stable cell lines were obtained, with SD03-23 showing the highest expression level.
- Protein purification using Protein A affinity column resulted in high purity (>98%) for all monoclones.
- The optimized stable cell line demonstrated satisfactory protein yield, achieving the project's goal.
Customer Testimonials
"Your AAV-producing stable cell lines with inducible promoters slashed our capsid optimization time by 50%. The combination of FACS-based monoclonality assurance and deep NGS profiling aligns perfectly with EMA/FDA guidelines for gene therapy commercialization."
Dr. Clara Schneider, Head of Bioprocess Innovation | Gene Therapy CDMO
"Profacgen’s glyco-engineered plant cell lines increased our recombinant protein yield by 6-fold for sustainable biopesticides. Their AI-driven metabolic flux analysis is redefining scalable agro-biomanufacturing."
Prof. Kenji Yamamoto, Director of Bioproduction | Agri-Bioscience R&D Hub
"The HEK293 suspension clones for spike protein production exceeded our titer expectations by 200%. Your serum-free media adaptation and plasmid-free integration were vital for pandemic-ready GMP manufacturing."
Dr. Anika Patel, CMC Lead | Next-Gen Vaccine Biotech
"Your GS-/- CHO platform with integrated QC reporters accelerated our bispecific antibody timeline by 40%. The end-to-end clonal stability data and ICH-compliant documentation streamlined our FDA pre-IND process."
Dr. Lucas Moreau, VP of Cell Line Development | Global BioPharma
"Custom yeast cell lines for terpenoid synthesis achieved 15x productivity gains. Profacgen's multi-omics-guided pathway optimization and HTP microfluidics screening are unmatched for industrial synthetic biology."
Dr. Sofia Herrera, Senior Scientist | Metabolic Engineering Startup
"Profacgen’s microbial strains for cellulase production tripled our industrial enzyme titers while cutting fermentation costs by 40%. Their high-throughput fermentation screening and ISO-compliant strain stability data accelerated our biofuel feedstock pipeline’s time-to-market."
Dr. Henrik Voss, CTO | Industrial Enzyme Manufacturing Group