Microscale Thermophoresis (MST)
Background
Introduction
Microscale Thermophoresis (MST) is this neat approach for diving into how molecules interact with each other. It keeps an eye on how the glow of a target molecule changes when you add different amounts of another molecule. This way, we get a sense of how strong their bond is. MST happens inside tiny tubes, letting us study molecules in conditions that mimic their natural surroundings. An infrared laser heats things up just a bit, creating a small temperature difference that makes the molecules drift in specific patterns. By watching these movements, we can figure out how they're mingling. What's really cool is that the molecules aren't stuck to anything, so they remain in their true form.
MST is becoming more popular because it's so precise and versatile. As technology improves, MST instruments are getting better at detecting even the smallest interactions. This makes them perfect for everything from basic research to developing new drugs. With more applications being discovered all the time, MST is definitely a technique to watch in the future.
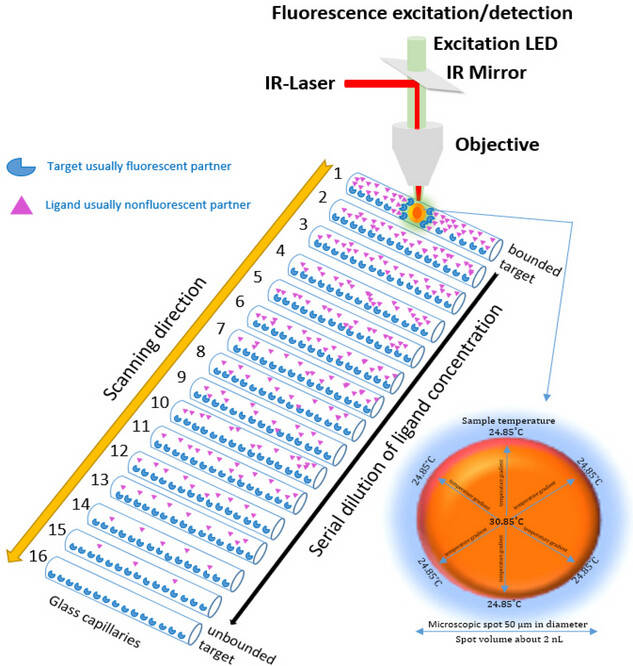 Fig1. Microscale thermophoresis (MST) process in a representative diagram. (Asmari. et al. 2023)
Fig1. Microscale thermophoresis (MST) process in a representative diagram. (Asmari. et al. 2023)
Applications of MST
- Single Dose Screening: MST can determine if a target and ligand interact through a single dose experiment.
- Steady-State Affinity: MST can determine the exact and true steady-state affinity of interactions (dissociation constant Kd).
- Protein Aggregation: MST can assess protein aggregation and its induction by ligand binding.
- Ligand Competition: MST can analyze the competitive effects of multiple ligands targeting the same protein.
Advantages of MST
- Minimal Sample Use: MST needs just a tiny bit of sample, which is great if you're working with rare or scarce materials.
- Flexible Buffers: You can run MST in all sorts of buffers, even those complicated biological ones.
- No Need to Immobilize: Since tests are done in solution, biomolecules stay in their natural state.
- Broad Concentration Spectrum: MST can examine interactions from picomolar to millimolar levels, offering a wide dynamic range.
- Quick and Efficient: MST experiments wrap up fast, so they're perfect for high-throughput screening.
Inquiry Now
Service Procedure
- Comprehensive Service Workflow:
- Deliverables:
- In-Depth Experimental Report:
We offer a thorough report that outlines the experimental setup, conditions, and analysis of MST data. You'll find essential details like binding affinity (KD), stoichiometry, and thermodynamic info, giving you a clear picture of how your biomolecules interact.
- Data Visualization:
Our package includes detailed graphs and charts displaying MST signals, dose-response curves, and binding interactions. These visuals make it easy to interpret the data and spot key trends and insights.
- Actionable Insights:
Beyond just the numbers, we provide expert opinions on the MST findings in line with your research objectives. You'll also get practical suggestions for additional experiments or optimizations, allowing you to make smart choices for your future steps.
- Service Details:
- High-Throughput Screening
We've got a top-notch MST setup with a 96-capillary system that lets you zip through multiple samples in no time. It's ideal for those big projects where you need to churn out lots of data fast. Whether you're hunting for new drugs or diving deep into protein interactions, our system ensures you get through your samples quickly without sacrificing accuracy.
- Versatile Assay Buffers
Our MST services are super flexible. You can run experiments in all sorts of buffers, even those complex biological fluids. This means you can keep your experiments as close to real-world conditions as possible, preserving the natural state of your biomolecules. No matter if you're working with proteins, nucleic acids, or small molecules, our system is ready to adapt to whatever you need.
- Real-Time Quality Control
We're all about making sure your data is spot-on. Our MST platform comes with real-time monitoring that keeps an eye on things like polymerization, precipitation, and sticking during experiments. This instant feedback lets us tweak things on the fly and troubleshoot any issues, so you end up with data you can trust every single time.
Inquiry Now
Why Choose Profacgen?
Advantages
- Low sample consumption: only 4µl is required per sample
- Free choice of assay buffers: biological liquids can also be used
- Short analysis times: fast and high throughput
- Real-time quality controls: online polymerization, precipitation and sticking control
- Readily allows competitive binding studies to be performed, and orthosteric and allosteric binders to be discovered
- No immobilization required: measurement is done in solution
- Wide temperature range: analysis can be performed from 20°C to 45°C
- Wide concentration range: Affinities can be analyzed in the pM-mM range
- Wide molecular size range: from 100 Da to 1 MDa
Case Study
Project: Microscale Thermophoresis (MST) Analysis of Protein-Ligand Interactions
Background
The project aimed to verify the in vitro molecular interactions between two proteins and four small molecule compounds provided by the customer. The goal was to determine the binding affinity of these interactions and obtain affinity data (KD values) for the protein-ligand pairs.
Results
- MST Traces and Fitting Plots:
- MST traces and Fnorm fitting plots were generated for the interactions between proteins and small molecule compounds.
- The point selection period was 1.5-2.5 seconds, indicating successful detection of binding events.
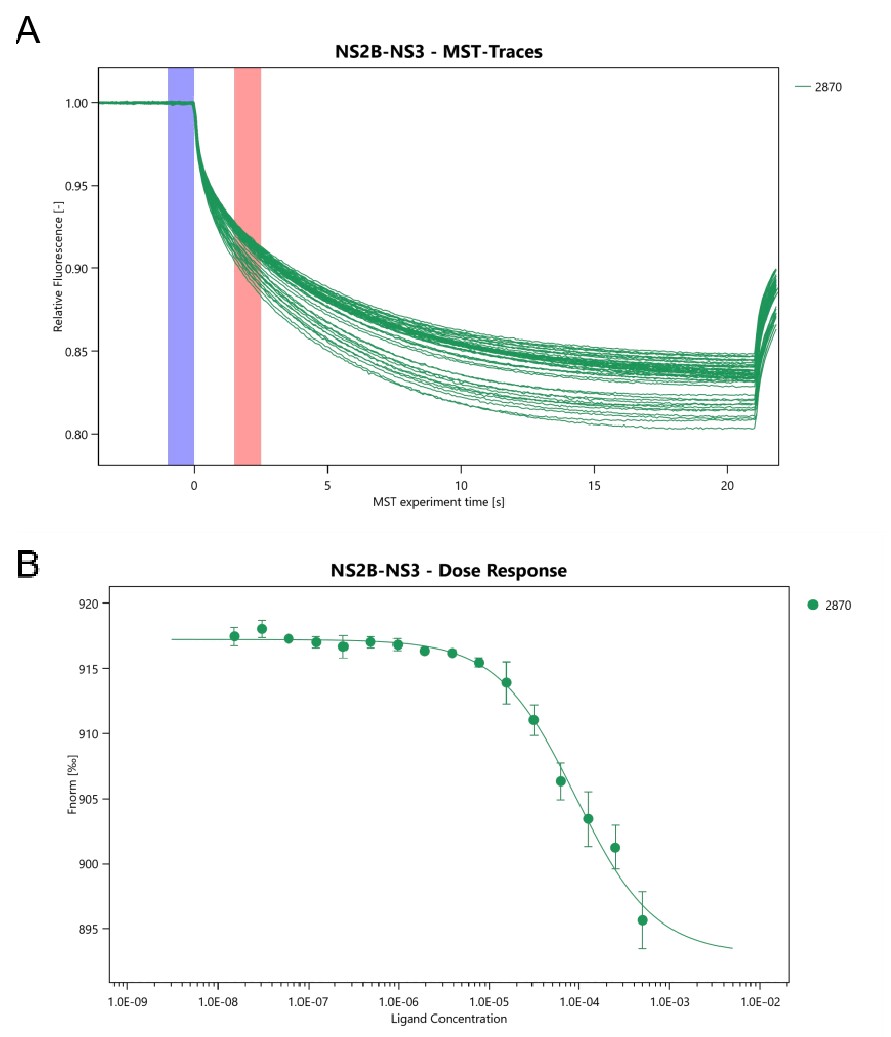 Fig2. Measurement of protein binding constants.
Fig2. Measurement of protein binding constants.
(A) MST Traces result. (B) Fnorm fitting plot.
- Binding Affinity Data:
- KD (Dissociation Constant) values were determined for each protein-ligand pair:
| Test Combination |
KD |
| A - C |
91.26 μM |
| A - D |
52.98 μM |
| B - E |
151.27 μM |
| B - F |
33.11 μM |
- Signal-to-Noise Ratio:
- The signal-to-noise ratio was 36.3, indicating high-quality experimental data (threshold > 5).
- Binding Characteristics:
- The protein migration rate increased with the concentration of the ligand, showing an "S" shaped curve with distinct upper and lower plateaus. This indicated specific binding interactions with medium binding strength.
Conclusion
The MST experiments successfully determined the binding affinities between the provided proteins and small molecule compounds. The results demonstrated specific interactions with measurable dissociation constants, providing valuable insights into the molecular interactions. The project achieved its goal of characterizing the binding affinities for the protein-ligand pairs, supporting further research in drug discovery and protein interactions.
FAQs
Q: Can MST work with limited sample amounts?
A: Absolutely. MST requires only small amounts of sample (nM concentrations and µL volumes), making it perfect for situations where samples are limited or precious. You can still obtain detailed results without needing large quantities.
Q: How long does an MST experiment typically take?
A: MST is designed for efficiency. Experiments are usually completed quickly, making it an excellent choice for high-throughput screening. Whether you have a few samples or many, MST delivers results rapidly.
Q: Is MST suitable for use with complex biological samples?
A: Absolutely! You can run MST in all sorts of buffers, even those tricky complex biological fluids. This makes it possible to perform experiments in conditions that are really close to what you'd find in nature, helping your biomolecules stay just as they are.
Resources
Reference:
- Asmari M.; et al. Studying molecular interactions via capillary electrophoresis and microscale thermophoresis: A review. Electrophoresis. 2023;44(13-14):1114-1142.
 Fig1. Microscale thermophoresis (MST) process in a representative diagram. (Asmari. et al. 2023)
Fig1. Microscale thermophoresis (MST) process in a representative diagram. (Asmari. et al. 2023)
 Fig2. Measurement of protein binding constants.
Fig2. Measurement of protein binding constants.