Ion Channel Screening Service
Background
Overview of Ion Channel Screening
Ion channels are pore-forming proteins that are located within the membrane of most cells and of many intracellular organelles. Their functions include maintaining a resting membrane potential, conducting action potentials and other electrical signals. They are characterized by their selective permeability - the ability to allow only certain sized/charged ions to pass through, thus they play vital roles in various physiological activities. These features make themselves become perfect targets in the process of novel drug development. Up to now, approximately 20% of the registered drugs are targeted for ion channels.
Ion channels play extensive biological roles in a wide variety of biological processes such as cardiac, skeletal and smooth muscle contraction, nutrient transportation and T-cell activation. There have been hundreds of different types of ion channels discovered so far. Ion channels can be classified into voltage-gated channels and ligand-gated channels based on gating, i.e. the stimuli that is responsible for opening and closing the channels. Ion channels can be also classified by their ion permeability, such as chloride channels, potassium channels, sodium channels, calcium channels, proton channels and non-selective cation channels. Besides, ion channels are also classified on the basis of subcellular localization, such as plasma membrane channels and intracellular channels.
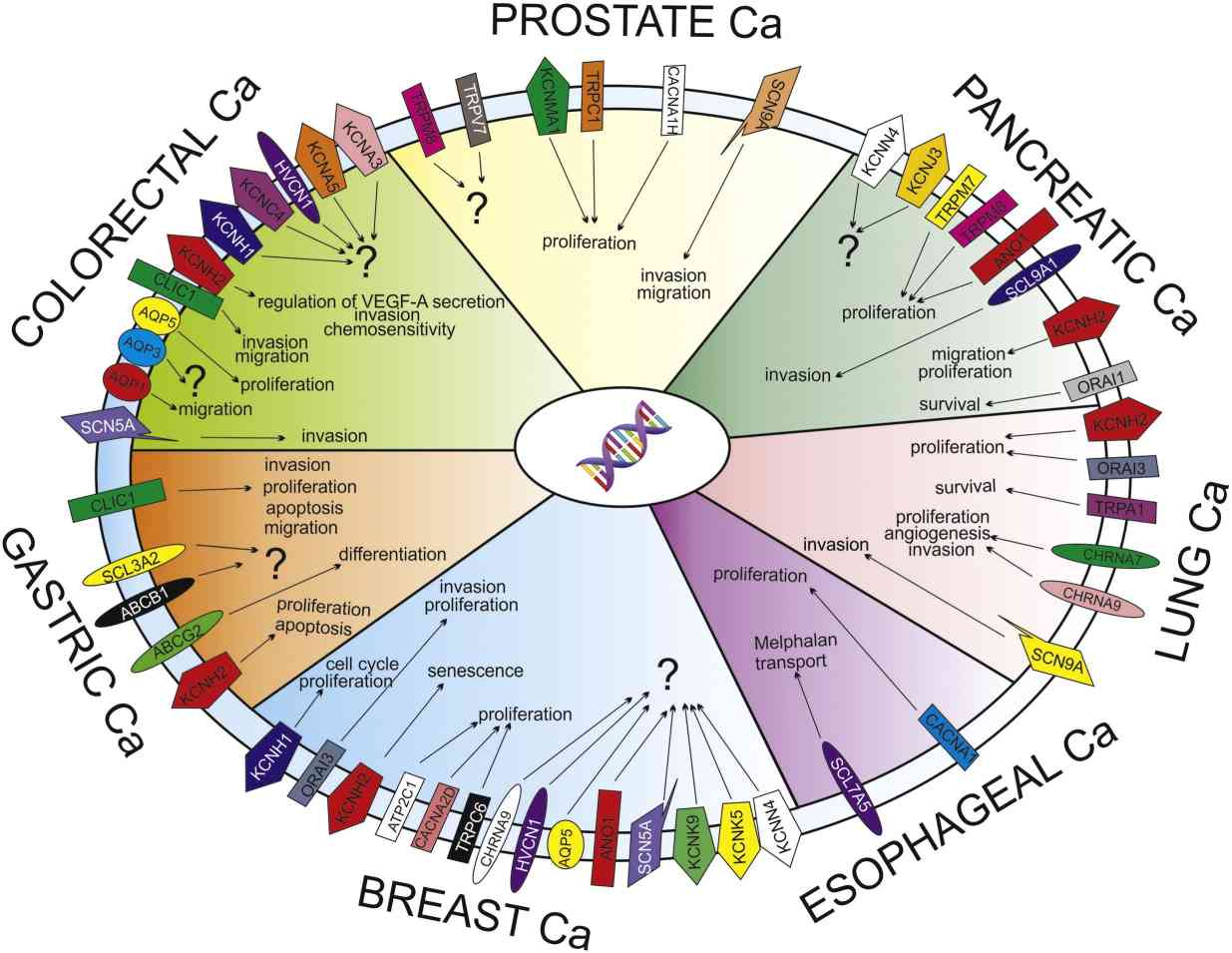
Fig1. Ion channels and transporters (ICT) profile and role in the solid cancers. (Lastraioli E, et al., Biochim Biophys Acta. 2015)
Applications of Ion Channel Screening
Ion channel screening is a key technology in drug discovery and development that covers a variety of ion channel types, including potassium channels, sodium channels, and calcium channels. It is precisely because of the diversity of channel types that the functional diversity of ion channels is created. The technology is used in a variety of fields, most widely in drug discovery and disease treatment.
 Disease correlation study
Disease correlation study
Ion channels have been linked to many diseases, including pain, cardiovascular disease, and neurological disorders. Through screening techniques, researchers can identify compounds that can regulate the activity of these channels and, in turn, develop drugs to treat related diseases.
 Drug safety evaluation
Drug safety evaluation
During drug development, evaluating the effects of drug candidates on cardiac ion channels is an important step in ensuring drug safety. For example, hERG potassium channels are closely associated with cardiac safety, and screening techniques can be used to assess whether compounds cause cardiotoxicity.
 Discovery of drug tool molecules
Discovery of drug tool molecules
Targeting newly discovered ion channels, such as the Piezo family, screening techniques can help find drug-tool molecules that can regulate the activity of these channels, providing a basis for further pharmacological research and drug development.
 Therapeutic strategy development
Therapeutic strategy development
By screening for modulators of specific ion channels, researchers can develop new therapeutic strategies for specific diseases, such as compounds that target potassium channels that may help treat conditions such as high blood pressure.
Service Procedure

Pipeline
Profacgen has developed a long list of ion channel assay models with good signal read-out and stability. Our ion channel assay models include sodium channels, TRP channels, potassium channels, calcium channels, P2X channels and other channels.
Profacgen offers both direct methods and indirect methods to help the study on your target ion channels. As a direct method, patch clamp is normally used in our lab, which is credited as a gold standard in ion channel research. As for indirect methods, we offer fluorescent dye based detection assays, in which fluorescent dye is loaded into cells and then analyzed by specialized plate readers. The readers can detect the concentration change of certain ions caused by ion flow through the channel. You can choose fluorescence assay combined with ion-work based high-throughput screening, or physiological characterization by conventional patch clamp electrophysiology or automated electrophysiology according to your specific requirements.
- Fluorescence assay combined with ion-work based high-throughput screening
Fluorescence based detection methods do not directly measure ion current, but rather measure membrane potential dependent or ion concentration dependent changes in the fluorescence signal due to ion flux. Our methods are used to monitor a wide range of biological activities such as molecular dynamics and interactions, enzyme activity, signal transduction, cell health, and the distribution of molecules, organelles, or cells, offering high sensitivity, a wide selection of fluorophores, simplicity of operation, and multiple reading modes.
- Physiological characterization by conventional patch clamp electrophysiology
Traditional patch-clamp technology is considered the "gold standard" for ion channel detection. This technique uses fine glass electrodes to form a tight seal with the cell membrane, allowing researchers to directly measure changes in the potential of the cell membrane and the current activity of ion channels. Profacgen offers a variety of different recording modes for this technology, such as cell attachment mode, whole cell mode, inside-out mode, and extrode-in mode, which you can choose to use for electrically excited cells, such as neurons and muscle cells, or for non-excitatory cells.
- Automated electrophysiology
This technique improves the throughput and efficiency of experiments by automating patch clamp electrophysiological measurements. We provide an automated platform to help you more quickly screen and identify ion channel modulators, which are critical to the function of ion channels in physiological processes such as nervous system signaling, electrolyte transport and muscle contraction.
Platforms
 |
ICR Technology The activity of ion channels can be detected by measuring the change of ion concentration inside and outside the cell, which is suitable for screening and analysis of various ion channels. |
 |
Fluorescence Detection System Fluorescence based detection technology can be used to monitor the activity of ion channels and is suitable for high-throughput screening. |
Our Advantages
- Fully Customized service
- Thoroughly validated high-throughput drug screening model
- Various detection methods
- Multiple testing panels
- Time & cost-efficient
Case Study
Case study 1: Mo S, et al., Front Genet. 2023
This study investigates the molecular mechanisms of insulinoma, a pancreatic neuroendocrine tumor, by analyzing differentially expressed ion channel-related genes (DEICRGs) from an mRNA dataset. Using protein-protein interaction and machine learning, researchers identified three key genes (MCOLN1, ATP6V0E1, ATP4A) and developed a diagnostic model with an AUC of 0.801. The genes were linked to insulin secretion and lysosome function, with inverse associations to the TGF-beta pathway. Immune infiltration differences were also observed.
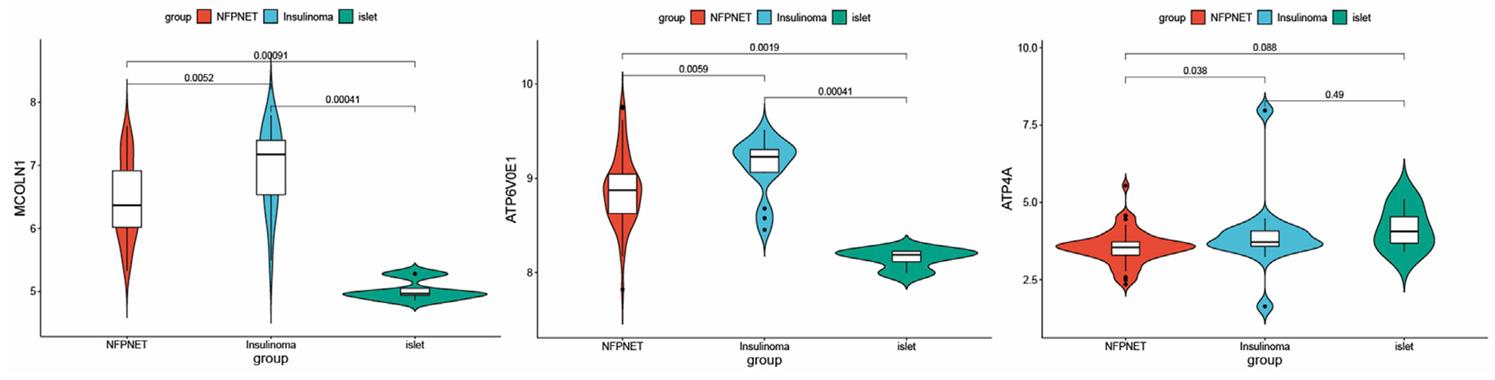
Fig2. mRNA expression of MCOLN1, ATP4A, and ATP6V0E1 in three different groups.
Case study 2: Liu P, et al., Research (Wash D C). 2024
Tinnitus, a common auditory issue, can lead to hearing loss and cognitive and psychological issues. It is associated with dysfunction of potassium channels KCNQ2 and KCNQ3 in the cochlear nucleus. This study explored their role in tinnitus and the potential of mid-infrared photons as a treatment. Using a noise-induced tinnitus model and various techniques, researchers found increased neuronal excitability in the auditory cortex linked to KCNQ2 and KCNQ3 dysfunction. Infrared photon irradiation reduced this excitability, inhibited excitatory neuron firing, and improved auditory responses in tinnitus-afflicted animals, suggesting a promising therapeutic approach.
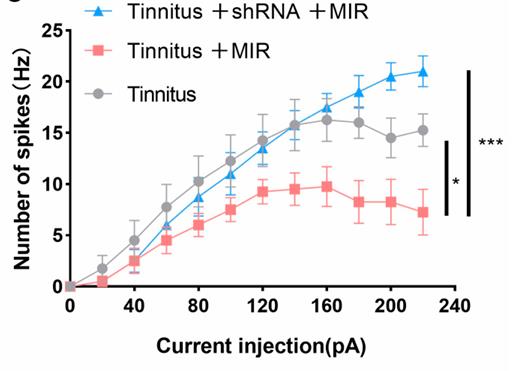
Fig3. Active properties of excitatory neurons for patch-clamp recording.
Case study 3: Niu H, et al., Nat Commun. 2024
Researchers discovered that a complex of Tmem63b and Slc19a2 proteins triggers phospholipid scrambling (PLS) in cells in response to calcium (Ca2+) changes. Using CRISPR screening, they found that Tmem63b initiates PLS, and this process is dampened by Tmem63b deletion but continuously active in disease-related mutants. Further screening revealed Slc19a2 and the Kcnn4 channel as regulators of PLS, with their absence reducing PLS activity. Tmem63b and Slc19a2 were shown to form a functional dimer, indicating that this complex, along with Kcnn4, mediates Ca2+-induced PLS.
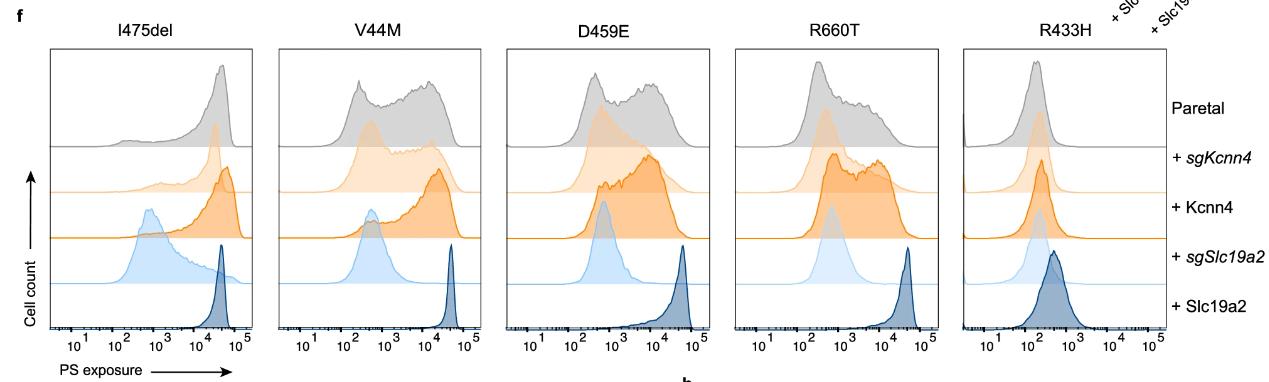
Fig4. PS exposure assay of Tmem63b mutants-GFP-expressing cells.
Resources
References
- Lastraioli E, et al. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015;1848(10 Pt B):2685-2702.
- Yu HB, et al. High throughput screening technologies for ion channels. Acta Pharmacol Sin. 2016;37(1):34-43.
- Gentzsch M, Mall MA. Ion Channel Modulators in Cystic Fibrosis. Chest. 2018;154(2):383-393.
- Mo S, et al. Identifying target ion channel-related genes to construct a diagnosis model for insulinoma. Front Genet. 2023;14:1181307.
- Liu P, et al. Mid-Infrared Photons Alleviate Tinnitus by Activating the KCNQ2 Channel in the Auditory Cortex. Research (Wash D C). 2024;7:0479.
- Niu H, et al. Phospholipid scrambling induced by an ion channel/metabolite transporter complex. Nat Commun. 2024;15(1):7566.
