Biomarker Discovery and Development Service
Profacgen supports your drug development needs by providing a full suite of biomarker discovery, development, biomarker assay verification and validation services as well as biomarker testing services (both large and small scale) across the drug development continuum, from early proof concept to commercial laboratory testing. With over 25 years of experience and industry leadership in developing and performing immunoassays on human and animal specimens, our biomarker analysis can accelerate your drug discovery, verification and validation programs.
Introduction of biomarker
Biomarkers, also called biological markers, are biochemical indicators that can be measured and evaluated the changes or the possibility of changes related to the functions or structures of system, organs, tissues, cells and subcellular. Biomarkers can be used in disease diagnosis, disease stages or used for evaluation of new drugs or treatment to assess the efficacy and safety in the target population. It can also help researchers put forward more effective ways of diagnosis and treatment, especially in cancer, cardiovascular disease, chronic diseases (such as diabetes, neurological disorders and complex), which has an important value on the prevention and control of diseases.
Biomarker discovery and development
Biomarker discovery refers to a process of discovering new protein biomarkers. There are several processes for the discovery of biomarkers: do biological experiments to get the raw data; convert the original data into numerical data for further analysis; then the data were preprocessed and visualized before statistical analysis; finally, big data methods such as machine learning and artificial intelligence were used to make statistical analysis of the data, and possible biomarkers were finally found. During biomarker discovery, the biomarker candidates need to be identified with high confidence, and simultaneous quantitation information provided, which can demonstrate the proteins that are in response to disease change in statistical degree.
Biomarker verification and validation
After biomarker discovery, the biomarker candidates discovered requires validation of bigger sample sets that covers extensive patient population. Because too many candidates to be validated may cause a potential bottleneck. For the purpose of avoiding this situation, verification as a step is used by screening potential biomarkers. It can make sure that the highest quality candidates from discovery step can be used for the expensive validation step. Also, verification step needs high throughput techniques and fewest preparation of samples, which can provide high specificity and sensitivity. The various stages of biomarker from discovery to validation with proper techniques are shown as figure1.
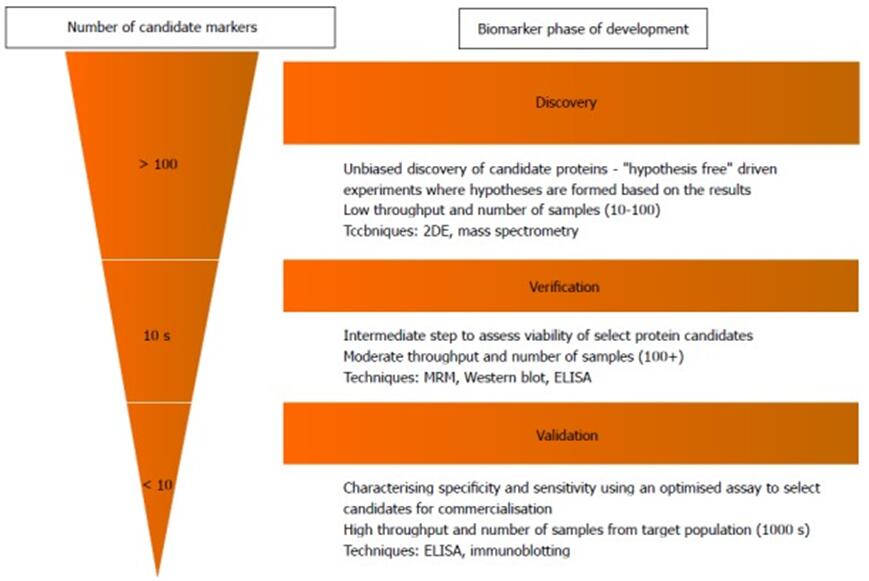
Figure1. The various stages of biomarker from discovery to validation with proper techniques.
Our service of biomarker discovery:
Discovery platforms: two-dimensional electrophoresis (2-DE), mass spectrometry (MS) or liquid chromatography coupled with multistage accurate mass spectrometry (LC-MSn) and stable isotope labeling by/with amino acids in cell culture (SILAC).
Data analysis: performance evaluation, statistical analysis, and multi-variant analysis and visualization.
Our service of biomarker verification and validation:
Verification and validation platforms: Multiple reaction monitoring (MRM) protein assay or liquid chromatography multiple reaction monitoring mass spectrometry (LC/MRM-MS), enzyme linked immunosorbent assays (ELISAs), Western blot.
Why choose our biomarker services for your research study?
| Expertise: Full access to all Profacgen products and development expertise. | |
| Quality: Stringent quality assurance program evaluates all study activities, including performance, control results, standard operating procedures, equipment, and personnel. | |
| Trust: Full accordance with the contracted study requirements. | |
| Rapid: Delivery of results and unsurpassed customer services. | |
| Flexibility: Flexibility to work on sponsor-directed studies to fulfill any specific requests for assay service. | |
| Experience: Proven service records for contract research organizations, academic groups, government organizations, and pharmaceutical companies. |
References:
1. Canary center at Stanford. Biomarker Discovery, Verification and Validation. [Online]. Accessed from: https://canarycenter.stanford.edu/core-facilities/proteomics/biomarker.html
2. P.PY.Chan, V.C.Wasinger and R.W.Leong.2016. Current application of proteomics in biomarker discovery for inflammatory bowel disease. World J Gastrointesr Pathophysiol. 7 (1): 27-37.