A New Enzyme for Remove Sialic Acids on Glycoproteins
Glycosylation plays a crucial role in protein folding, trafficking, stability, and cellular activities such as receptor binding, cell signaling, immune recognition, inflammation, and pathogenesis. N-glycosylation and O-glycosylation are the two primary forms of glycosylation modification. N-linked glycans are linked to the amide side chains of asparagine (Asn) residues on the protein with a core; in contrast, O-linked glycans have a disaccharide core of Core 1 or Core 3 attached to the hydroxyl side chains of Ser or Thr residues.
The terminal residues on these N- or O-glycan chains are usually sialic acids. An essential first step in glycosylation studies of glycoproteins is usually the removal of all non-reducing terminal sialic terminal acid residues. The sialic acid at the end has a severe impact on the function of the antibody. The higher the content of sialic acid, the lower the affinity of IgG and Fc receptors. The reason may be that the large space occupied by sialic acid reduces the elasticity of the hinge region of the antibody, thereby reducing the receptor binding ability. The level of sialylation may affect the metabolism of protein drugs, so desialylation is very important.
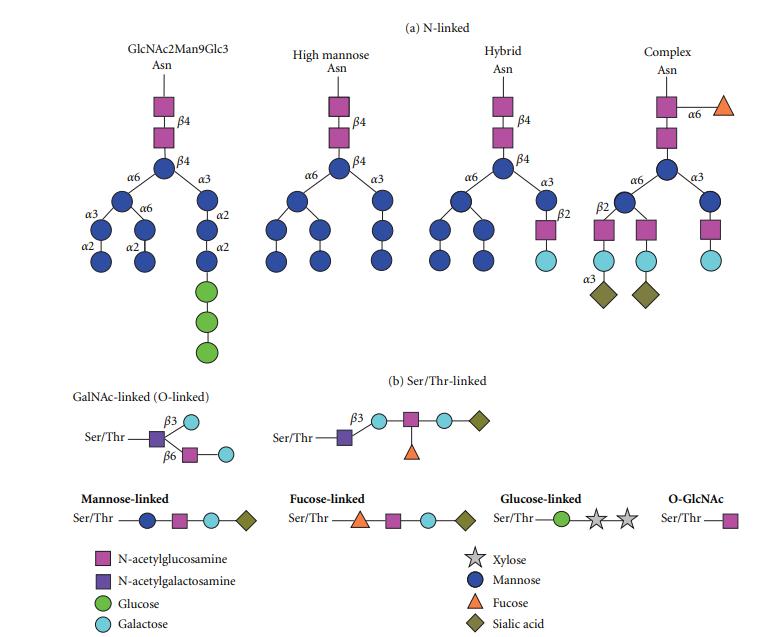 Fig.1 Common N- and O-linked glycans.
Fig.1 Common N- and O-linked glycans.
This new enzyme is a kind of sialidase that can remove and analyze sialic acids on the glycoproteins and release glycans. This new enzyme product cannot only complete the removal of α2-3, α2-6, and α2-8 sialic acids but also can specifically completely remove α2-3 sialic acids. This enzyme is derived from Akkermansia muciniphila and expressed in the E.coli strain. It is a crucial tool enzyme for glycosylation research and can be widely used to analyze glycoproteins and glycolipids.
Advantages
- Unique activities and work faster
- Co-factors are not needed
- Active on both N-linked glycan and O-linked glycan
- Removes all sialic acids in a shorter time
- High purity with no contaminating proteases and other glycosidases
- High stability
Potential Applications
- Researches of glycosylation modification
- Eliminate glycoprotein heterogeneity
- Analysis of Glycan Structure
- Cancer research and identification and in vitro diagnostics of abnormal sialylation
Profacgen is a company dedicated to research in the biological field. We have been committed to discovering advanced experimental techniques to provide stronger support for our customers' research. Please do not hesitate to contact us for more details if you are interested in this new method, and we will provide a considerate service for you. At the same time, we also provide other protein interaction detection methods, please move to our website for more details in protein-protein interaction.
Reference
- Evelyn H Kim, Davud E Misek, Glycoproteomics-Based Identification of Cancer Biomarkers, International Journal of Proteomics, https://doi.org/10.1155/2011/601937.