Immunogenicity testing is a crucial part of biopharmaceutical development. More stringent regulations regarding immunogenicity assay performance necessitates the development of assays exhibiting excellent sensitivity, precision, free drug tolerance and minimal matrix effects. Immunogenicity assays can be applied in detection and confirmation of anti-drug antibodies (ADAs) and neutralizing antibodies (NAbs) supporting nonclinical and clinical studies.
The development and validation of immunogenicity assays is under constant discussion within the industry. Based on our GLP-compliant service laboratories, Profacgen is involved in international workshops and has R&D programs to keep ahead of the latest developments in this area, so that we can best help our customers to accelerate their drug discovery and development programs.
Immunogenicity assays can be challenging due to interferences originating from the sample matrix, drug interference or target interference. In order to design a robust immunogenicity assay, it is important to start with the appropriate assay platform and high-quality reagents. There are two predominant strategies for proceeding through a biosimilar immunogenicity program based on the use of a single assay or two separate assays.
A single assay
One strategy to use for the immunogenicity program of a biosimilar is to employ one assay for the testing of all samples. The assay is developed for both products and a limited set of parameters in validation should be evaluated including screening cut-point, specificity cut-point, precision, and drug tolerance for both products in order to ensure that the assay is capable of detecting antibodies to both the biosimilar and the reference product.
Two assays
The use of two assays for the assessment of immunogenicity for a biosimilar program will enable the direct comparison of immunogenicity rates for the biosimilar and the reference product. The use of this approach entails a full validation of two separate assays, one for the detection of antibodies generated to the biosimilar and one for the detection of antibodies generated to the reference product. This strategy is most useful when looking for an interchangeability designation.
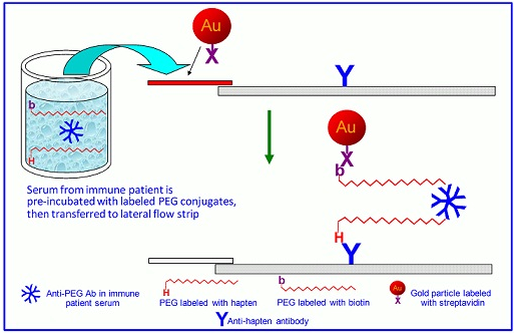
Figure 1. A general schematic of rapid immunogenicity tests.
Profacgen offers following immunogenicity testing services.
| Ligand Binding Assay | |
| Cell Based Assays | |
| Total Antibody Assay | |
| Neutralizing Antibody Assay | |
| Particle Size Characterization | |
| Critical Reagent Characterization | |
| Protein Aggregation Analysis |
Profacgen’s immunogenicity team has developed and validated in vivo and in vitro assays for many of our biopharmaceutical clients to screen and quantify immunogenicity caused by anti-drug antibodies (ADAs) and impurities. Based on the risk profile of the biotherapeutic protein or antibody and its intended use, our team will work closely with our clients to develop a custom panel of immunogenicity assays. Please do not hesitate to contact us for more details about our membrane protein modeling service.
Fill out this form and one of our experts will respond to you within one business day.