Serum IgG fusion service
Serum immunoglobulin (Ig) fusion protein refers to the recombinant protein with two domains, which connects the target protein gene with some Ig fragment genes. This method is suitable for protein or polypeptide drugs to prolong the half-life, so as to achieve a longer action time in the body. Ig fusion protein has many characteristics, firstly, the introduction of heavy chain stability region can significantly prolong the half-life of the fusion protein, which is more suitable for the application of recombinant protein in vivo. Besides, Fc segment can cross placenta, bind to complement and mediate complement dependent cytotoxicity, bind to Fc receptor and mediate ADCC effect. In addition, the Fc domain of IgG, IgM and IgA can form polymeric molecules, which makes the recombinant protein have stronger antigen binding force.
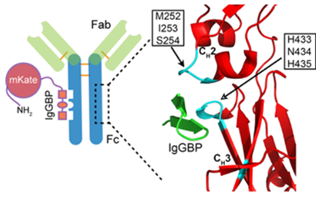 Figure 1. IgGBP fusion as a strategy to improve protein half-life by targeting serum IgG (Baldwin III W M, et al. 2019).
Figure 1. IgGBP fusion as a strategy to improve protein half-life by targeting serum IgG (Baldwin III W M, et al. 2019).
The half-life of immunoglobulin subclasses IgG1, IgG2 and IgG4 in vivo is 2 ~ 3 weeks, and can be connected with most peptide and protein drugs. Therefore, it is very suitable to be used as a carrier to prolong the half-life of peptide or protein drugs. At Profacgen, we can provide different fusion strategies for our customer.
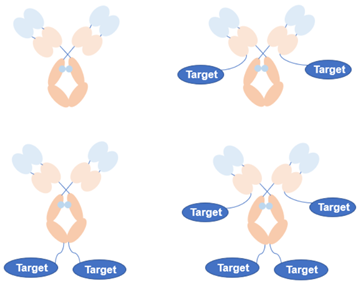 Figure 2. Structure of serum IgG fusion protein
Figure 2. Structure of serum IgG fusion protein
There are several benefits that Serum IgG fused to polypeptides or other therapeutics:
- Prolong the half-life for protein and polypeptide therapeutics;
- Improve the pharmacokinetic and pharmacodynamic characteristics of drugs and improve the bioavailability;
- Have a variety of fusion strategies based on light chain or heavy chain of IgG.
With our drug development platform, Profacgen can develop Serum IgG fusion methods for our customer. If you are interested in our services, please feel free to contact us. We are looking forward to cooperating with you.
References
- Baldwin III W M, Valujskikh A, Fairchild R L. The neonatal Fc receptor: Key to homeostasic control of IgG and IgG‐related biopharmaceuticals[J]. American Journal of Transplantation, 2019, 19(7): 1881-1887.
- Sockolosky J T, Kivimäe S, Szoka F C. Fusion of a short peptide that binds immunoglobulin G to a recombinant protein substantially increases its plasma half-life in mice[J]. PloS one, 2014, 9(7): e102566.
- Jo M, Ko S, Hwang B, et al. Engineered human FcγRIIa fusion: A novel strategy to extend serum half‐life of therapeutic proteins[J]. Biotechnology and bioengineering, 2020, 117(8): 2351-2361.
- Saunders K O. Conceptual approaches to modulating antibody effector functions and circulation half-life[J]. Frontiers in immunology, 2019, 10: 1296.