Bio-Layer Interferometry (BLI)
Background
Introduction
Bio-Layer Interferometry (BLI) is a modern, label-free optical method that lets researchers study biomolecular interactions in real time. Created by ForteBio, this tech helps measure and understand how proteins, nucleic acids, and other molecules interact, both in terms of quantity and quality. BLI works by tracking changes in the interference pattern of white light bouncing off a biosensor. As molecules attach or detach from the protein layer on the sensor, it detects shifts in thickness. This gives useful details about binding strength, speed, and specificity, making it a go-to tool for drug discovery, antibody development, and immunology research.
At Profacgen, we're all about giving researchers the best tools to study these interactions. Our BLI service covers everything from measuring protein levels to figuring out binding specifics, affinity, and kinetics. We're here to deliver top-notch data and clear insights, helping scientists move faster and make breakthroughs in life sciences.
How BLI Works
Bio-Layer Interferometry (BLI) is a no-label, real-time technique to track biomolecule interactions. It detects thickness changes in a biological layer on a sensor. BLI uses a fiber-optic biosensor with a coating that grabs proteins, antibodies, or nucleic acids. When you add a sample with an analyte, binding events shift the layer's thickness. This shift is detected by looking at how white light reflects off the sensor. By measuring these changes in real time, BLI gives you valuable info about how fast, how strong, and how specific the binding is.
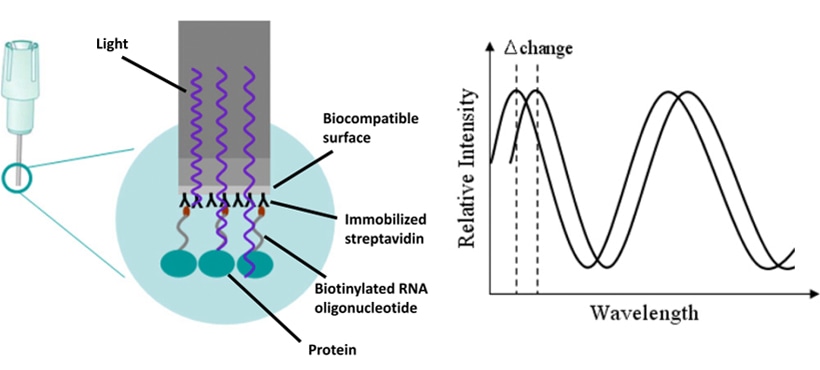 Fig1. Schematic representation of the principle of the BLI system. (Roh. et al. 2011)
Fig1. Schematic representation of the principle of the BLI system. (Roh. et al. 2011)
Key Features of the BLI System
High flexibility allowing quantitation ranging from ng/ml to mg/ml
Label-free and real-time analysis
Rapid test that enables high process efficiency and productivity
Relatively low cost
Crude sample compatibility (Insensitivity to pH of the matrix or changes in refractive index)
Advantages of BLI
Label-Free and Real-Time
BLI provides label-free detection and real-time monitoring, preserving biomolecule integrity and accelerating data acquisition.
High Throughput and Efficiency
BLI handles high-throughput screening and multiple samples at once, cutting down wait times and boosting efficiency.
Versatility and Sensitivity
BLI works with a variety of biomolecules and even messy samples, offering super-sensitive detection for low-concentration work.
Cost-Effective and Low Maintenance
BLI systems are low-maintenance and cost-effective, making them a smart pick for long-term projects.
Tailored to Your Needs
BLI allows for customizable biosensor surfaces and experimental setups, adapting to diverse research needs.
Service Procedure
- Comprehensive Service Workflow:
- Deliverables:
- Detailed Experimental Report
Summary of experimental design and conditions.
Comprehensive data analysis, including binding kinetics (association and dissociation rates), affinity constants (KD), and concentration measurements. - Data Visualization
Graphs and charts illustrating binding interactions and kinetic profiles.
Clear visual representation of experimental results. - Actionable Insights
Interpretation of data in the context of your research goals.
Recommendations for further experiments or optimizations.
- Detailed Experimental Report
- Service Details:
- High-Throughput Screening
We get that you need results fast. Our BLI platforms can handle a bunch of samples all at once, which is just what you need for big projects like antibody screening or drug discovery. Doesn't matter if you have a few samples or a ton, we'll make sure everything runs smoothly and efficiently. - Versatile Sample Compatibility
We handle all types of samples, whether it's proteins, nucleic acids, lipids, or small molecules. Even if your samples aren't in perfect shape or you're short on quantity, we can analyze them without any fuss. This kind of flexibility means we can cover a wide array of research needs. - Expert Support and Real-Time Analysis
We'll stick with you from start to finish. Our team will work closely with you to set up experiments that match your goals. And with our label-free, real-time BLI technology, you'll instantly see how your molecules are interacting. This helps you make quicker decisions and keeps your research moving forward.
- High-Throughput Screening
Why Choose Profacgen?
Our BLI service advantages:
- Best price in the market
- Short turn-around time
- Consistent analysis results
- Highly experienced team
Case Study
Background
The customer required an analysis of the binding affinity between a protein and its ligand. This project utilized Bio-Layer Interferometry (BLI) to determine the binding affinity. Two samples were provided by the customer, with the ligand being a protein that needed to be immobilized on biosensors, and the analyte being the XXX protein. The goal was to determine the equilibrium dissociation constant (KD) between the ligand and analyte.
Methods and Materials
Samples were provided by our customer. Other reagents and use of instrument were provided by Profacgen.
Total 2 Samples were provided by Customer.
| Ligand | Analyte | |
| Sample ID | Protein | XXX |
| Concentration | 50 ug * 2 | 200 ug |
| Any Tags | ------- | none |
| Purity | >95% | >95% |
| Storage temp. | -80°C | -80 |
| Hazardous/BSL level | 1 | 1 |
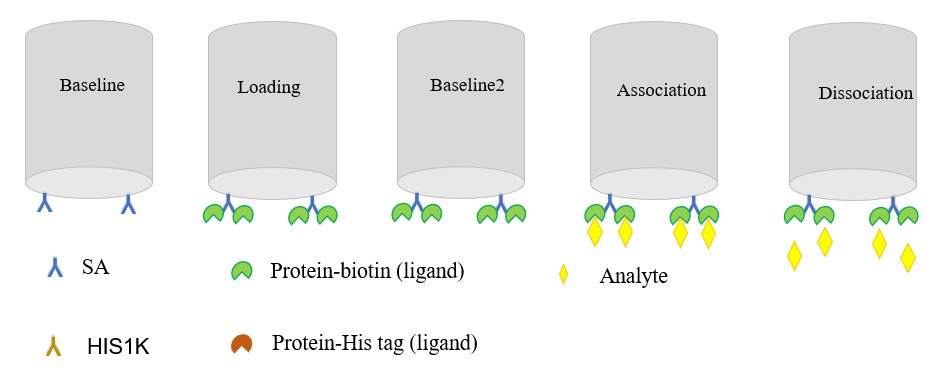 Fig2. Workflow for BLI method.
Fig2. Workflow for BLI method.
Results
Evaluation of the binding affinity of analyte to protein. The equilibrium dissociation constant (KD Value) was 7.21×10-8 M.
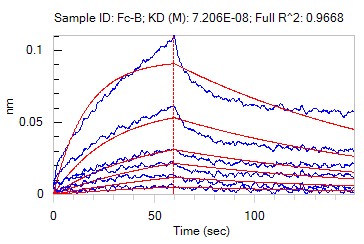 Fig3. Sensorgram curves for protein binding of Fc-1 in BLI-kinetic assay.
Fig3. Sensorgram curves for protein binding of Fc-1 in BLI-kinetic assay.
Conclusion
Here we report successful completion of the project. The BLI binding affinity result was as follows.
| Ligand | Analyte | Analyte Conc.(μM) | KD (M) | Kon (1/Ms) | Kdis (1/s) | RMax | R^2 | Fit Model |
| Protein | Fc-1 | 500-15.625 nM | 7.21E-08 | 1.09E+05 | 7.83E-03 | 0.1065 | 0.9668 | 1:1 binding |
FAQs
Resources
| Surface Plasmon Resonance (SPR) Service | Co-Immunoprecipitation (Co-IP) | Proximity-dependent Biotin Identification (BioID) Service | Yeast Two-Hybrid Screening | Pull Down Assay |
Reference:
- Roh C.; et al. Label free inhibitor screening of hepatitis C virus (HCV) NS5B viral protein using RNA oligonucleotide. Sensors (Basel). 2011;11(7):6685-6696.