Destabilization Domains (DDs)
Designing small-molecule ligands for a protein can be challenging and time-consuming as the process usually involves multiple rounds of screening and structural modification. Thus, it is beneficial to have a hybrid system that takes advantage of the simplicity of small-molecule ligands and the specificity of genetic approaches. Destabilization domains (DDs) represent a fusion protein component that is intrinsically unstable and destabilizes other proteins upon incorporation, leading to protein degradation. A well-known example of DDs is the Shield system, which incorporated a rampamycin-binding protein (FKBP12) into proteins as a build-in destabilizing domain to cause protein degradation in cells (Figure 1). However, when a rampamycin analogue (Shield1) is added to bind the destabilizing domain, the fusion protein can be stabilized and returns expression to a normal level. This approach enables the regulation of secreted proteins and their biological activity, which opens up new avenues for various applications such as cancer therapy and targeted gene delivery.
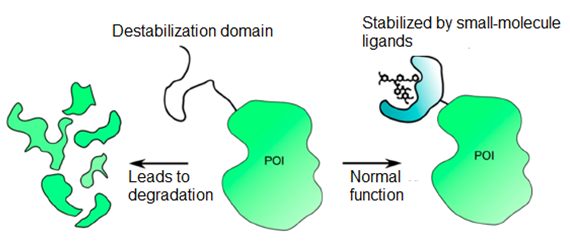 Figure 1. Mechanism of Destabilization Domains
Figure 1. Mechanism of Destabilization Domains
Over the past decade, a number of strategies based on DDs were discovered to harness the power of both small molecules and ligand-binding proteins, enabling temporal control of protein functions both in vitro and in vivo. Current research is focused on the development of similar strategies for a wider scope of target proteins, and with improved specificity. Unlike PROTACs, the use of DDs employs genetic encoding of the destabilization domain, which allows for higher level of specificity as well as precise control of the system. It should be noted that, however, incorporation of DDs into the protein of interest may induce function change and activity loss of the fusion protein, thus extensive method validation is usually necessary.
Indeed, the development of a successful DD system requires a high level of expertise in several related fields such as molecular biology, synthetic chemistry and protein engineering. At Profacgen, our scientists with diverse research backgrounds work closely to form a dynamic team with a focus on solving multidisciplinary problems. With years of experience in serving the pharmaceutical industry, we are committed to assist clients in virtually every aspect of therapeutics development and provide the most up to date technologies for our clients. Recently, the use of small-molecule agents to exert control over cellular protein levels has begun to show its great potential to generate both novel therapeutics and tools for studying biological systems.1 Relying on the advanced protein engineering platform and a large arsenal of in-house ligands and synthetic linkers, Profacgen is now offering the featured one-stop service for DD development to meet your diverse needs, including:
- Ligand synthesis, screening and validation: we design, synthesize and evaluate ligands based on known structures. In silico development is also available with our computational drug design station;
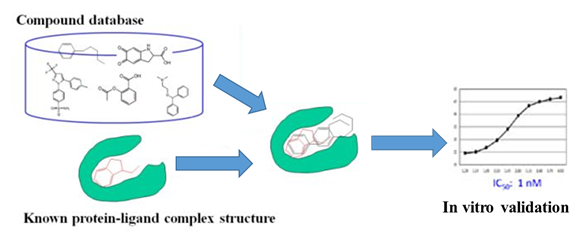 Schematic Representation of in Silico Ligand Discovery
Schematic Representation of in Silico Ligand Discovery
- Protein fusion: inherently unstable domains can be fused into any partner proteins of your interest at our state-of-the-art facility to greatly accelerate your research projects;
- In vitro and in vivo testing: function and activity of the newly generated destabilizing domain can be tested in solutions, living cells and animal models.
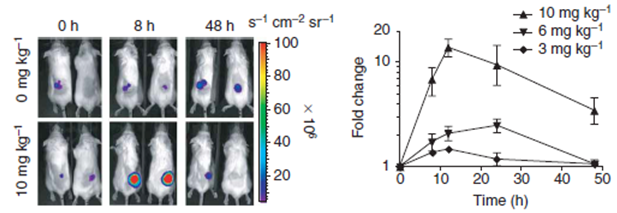 Example of Destabilization Domains Dsed in Cancer Research
Example of Destabilization Domains Dsed in Cancer Research
For more information regarding Profacgen's Destabilization Domains service, please contact us. Our customer service representatives are available 24 hours a day, Monday through Friday, to assist you.
Reference:
Banaszynski, L. A.; et al. Chemical control of protein stability and function in living mice. Nature Medicine 2008, 14, 1123.