Abnormal protein expression or activities are closely associated with many diseases, especially cancer. Therefore, down-regulating the targeted proteins is an effective strategy to treat diseases. Recently, a series of targeted protein degradation methods have been developed by using small molecules. protein degradation targeted chimeras (PROTACs) technology has also risen as a powerful tool to degrade target proteins by hijacking the ubiquitin-proteasome system that includes E3 ligases.
Target proteins are usually the proteins whose overexpression or accumulation may play an important role in the occurring of diseases. Many PROTACs have been developed to degrade the target proteins, including kinases (such as CDK, KARS, MEK, and Bcr/Abl), transcription factors (such as p53, STAT, RAR, ER, and AR), epigenetic tools (such as HDAC and BET bromodomain), and E3 ligase themselves (such as MDM2).
E3 Ligases are important components of the Ubiquitin Proteasome System (UPS). As the master regulator of protein homeostasis, UPS is essential in signal transduction, mitophagy, DNA damage repair, inflammatory pathways, endocytosis and cell-cycle progression. More than 600 E3 ligases have been discovered, and only a few of these have been used in PROTAC designs, which include Von Hippel-Lindau disease tumor suppressor protein (VHL), the Cellular Inhibitor of Apoptosis (cIAP), the Mouse Double Minute 2 Homologue (MDM2), and ereblon (CRBN). The development of anti-cancer PROTAC mainly uses the ligands of CRBN, VHL, MDM2, IAPS, dCAF15, dCAF16, RNF4 and RNF114 E3 ligases.
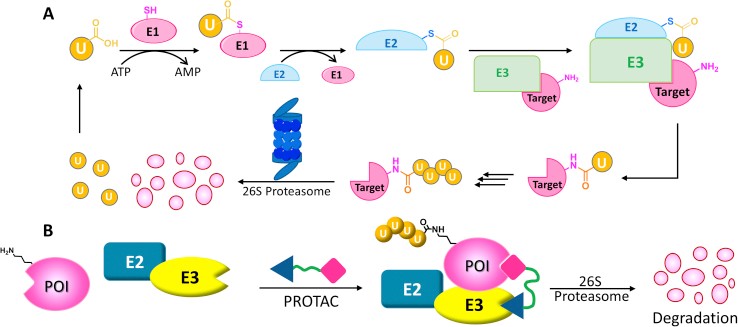
Fig1. The ubiquitin−proteasome system and PROTAC. (Wang Y, et al., Acta Pharm Sin B. 2020)
Development services for E3 ubiquitin ligases and their target proteins play an important role in multiple application scenarios, especially in the field of drug development and disease treatment. As research continues to deepen, more innovative applications are expected to emerge.
E3 ubiquitin ligase plays an important role in the regulation of cell cycle, DNA damage repair and apoptosis. By targeting specific E3 ubiquitin ligases or their target proteins, new therapies can be developed to treat cancer. For example, with PROTACs technology, degradation of specific carcinogenic proteins can be induced to inhibit tumor growth.
E3 ubiquitin ligase is a popular target for drug development. By screening and optimizing small molecule ligands, it is possible to develop compounds that can regulate the activity of E3 ubiquitin ligase, thereby affecting the stability and function of their substrate proteins. These compounds can be used as potential drug candidates to treat a wide range of diseases.
Researchers are exploring new E3 ubiquitin ligases to expand the application of PROTAC technology. By systematically analyzing the chemical ligand capabilities, expression patterns and protein-protein interactions of E3 ubiquitin ligase, we can provide new E3 ligase options for PROTAC design.
With the in-depth understanding of E3 ubiquitin ligase and its mechanism of action, more accurate treatment methods can be developed, such as the specific degradation of disease-related proteins through PROTACs technology to achieve precise intervention in diseases.
As an experienced service provider in biological research and drug discovery, Profacgen provides E3 ligase development including single-subunit E3 ligases, multi-subunit E3 ligase complexes, tissue specific E3 ligases, and other target proteins development services. With the comprehensive advanced platform, Profacgen offers our clients a one-stop PROTAC development services.
The study of protein ubiquitination plays an important role in cell biology and drug discovery. Profacgen offers various ubiquitin services including in vitro ubiquitin conjugation reactions, assay to measure protein ubiquitination in living cells and ubiquitin identification and quantification by proteomics analysis to help our customers to accelerate research progress and obtain better results.
With our phage screening platform and small molecule library, we can provide a novel E3 ligases discovery service and used in PROTAC design. Phage display is a powerful selection technique that allows for the rapid selection of proteins with desired properties including increased affinity, specificity, stability etc. Here we can immobilize our E3 ligand and screening a higher affinity E3 ligase.
Profacgen offers a comprehensive protein expression service to produce E3 ligases or target proteins from many different host systems for research, functional assays, target validation, or antibody development. The work flow of the expression and purification services includes Bioinformatics analysis, Cloning, Protein expression, Protein purification and other options.
A PROTAC molecule is composed of two covalently interconnected ligands, one to target protein and the other to an E3 ubiquitin ligase, with a linker of mostly 5-15 carbon or other atoms. The major challenge for PROTAC is that it is difficult to predict the activities of a PROTAC in different cells types, tissues or species. Thus, Profacgen is capable of offering one-stop PROTAC based on comprehensive and stable platform including in vitro evaluations of chemistry, cell biology, toxicities, dosage and efficacy studies.
Case study 1: Pao KC, et al., Nature. 2018
Ubiquitination begins with E1 enzymes activating ubiquitin, which is then transferred to E2 enzymes, forming E2-Ub. RING-type E3 ligases recruit E2-Ub and transfer ubiquitin directly to target proteins. HECT E3s form a covalent complex with E2-Ub before transferring ubiquitin. RBR E3s, featuring a RING domain and a catalytic cysteine, use a hybrid mechanism. This study reveals MYCBP2, a neuron-associated E3 ligase, as a unique RING-linked E3 with threonine-specific esterification activity. MYCBP2 utilizes two catalytic cysteines for ubiquitin transfer via thioester intermediates. The crystal structure of this RCR-type E3 ligase offers insights into its mechanism and threonine selectivity.
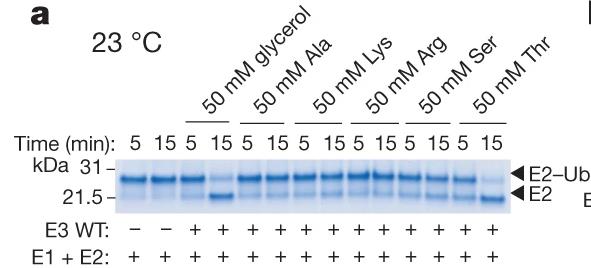
Fig2. E3-mediated multiple-turnover discharge reaction for a panel of amino acids (50 mM).
Case study 2: Karim M, et al., mBio. 2020
Viral protein functions, crucial for infection success, often result from posttranslational modifications (PTMs). In influenza A viruses (IAVs), PTMs regulate viral protein activity, but the PB2 replication protein's modifications, aside from degradation, are largely unknown. Researchers discovered that PB2 is nonproteolytically ubiquitinated during IAV infection, with E3 ubiquitin ligases CRL4s (based on cullin 4) driving this process. This ubiquitination is essential for viral replication and production. CRL4s interact with PB2 via DDB1 and DCAF receptors without affecting viral transcription or replication. They catalyze distinct lysine ubiquitination patterns on PB2, with K29-linked chains being predominant, representing a novel role for this ubiquitin linkage in viral infection regulation. Mutations in these lysines reduce viral production, highlighting the importance of CRL4-mediated PB2 ubiquitination in IAV infection.
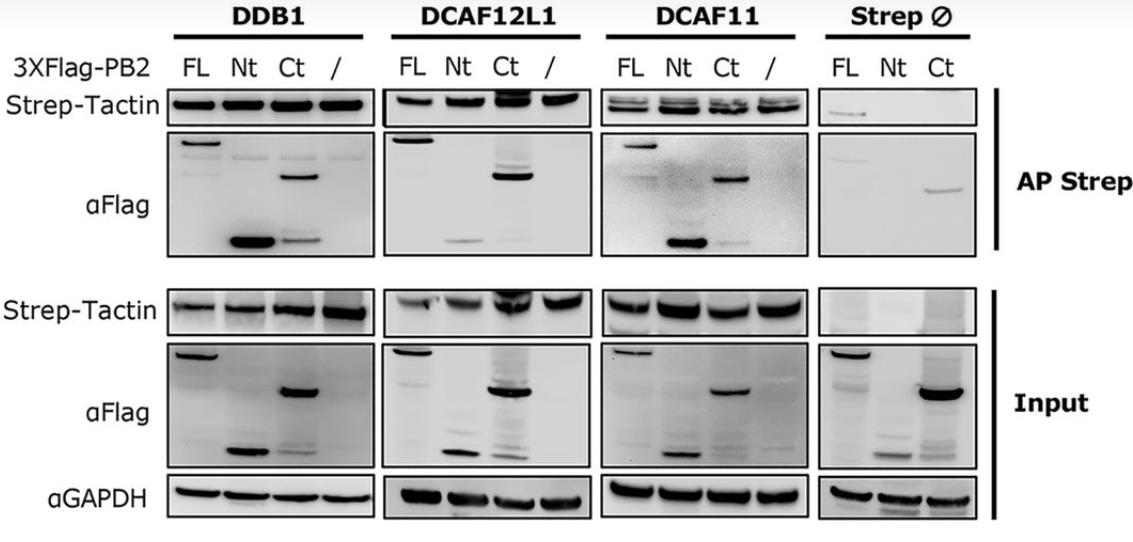
Fig3. Interaction domains of PB2 with the CRL4 factors.
Case study 3: Toyofuku T, et al., EMBO J. 2020
LRRK2 gene mutations are a leading cause of hereditary Parkinson's disease (PD), with mitochondrial dysfunction implicated in its pathogenesis. LRRK2 influences the connection between the endoplasmic reticulum (ER) and mitochondria, which is vital for mitochondrial energy production. LRRK2 affects E3 ubiquitin ligases MARCH5, MULAN, and Parkin through its kinase activity, influencing their interaction and regulation. The active form of LRRK2(G2019S) detaches from these ligases, triggering their activation by PERK phosphorylation and increasing the degradation of proteins that link the ER to mitochondria. Conversely, the kinase-dead LRRK2(D1994A) variant retains these ligases, preventing their activation and enhancing the levels of ER-mitochondrial tethering proteins. This LRRK2 function in ER-mitochondrial interactions is a significant determinant of cellular health and PD development.
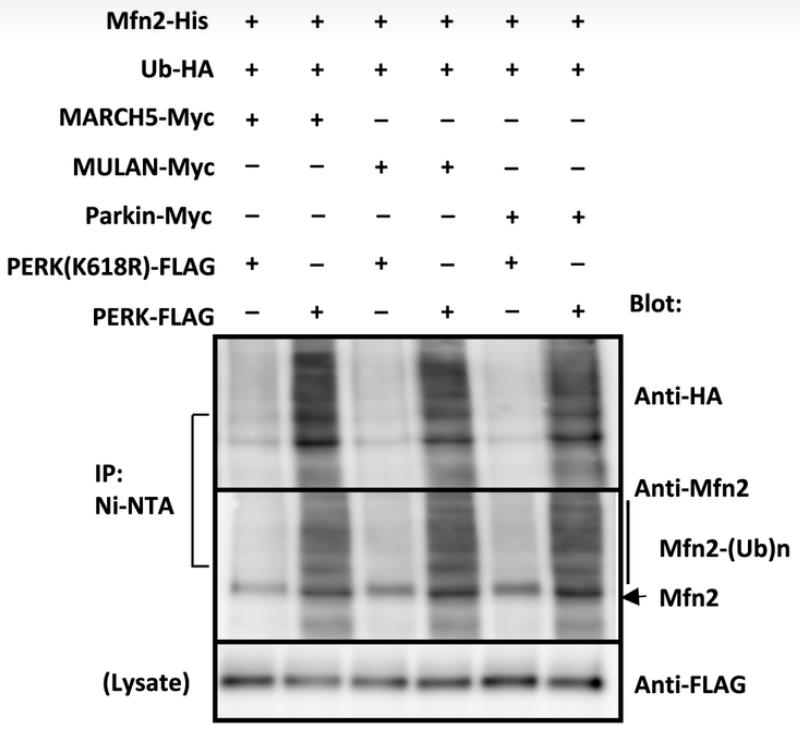
Fig4. In vitro ubiquitination assay using phosphorylated E3 ubiquitin ligases, HA‐tagged Ubl, and His‐tagged mitofusin 2 in the presence of E1 enzyme, UbcH7, and ATP.
References
Fill out this form and one of our experts will respond to you within one business day.