Fluorescence Resonance Energy Transfer(FRET)
Fluorescence resonance energy transfer (FRET) is an early developed technology. With the development of green fluorescent protein (GFP) application technology, FRET has become a powerful tool to detect the nano scale distance and the change of nano scale distance of biological macromolecules in vivo Immunoassay has a wide range of applications.
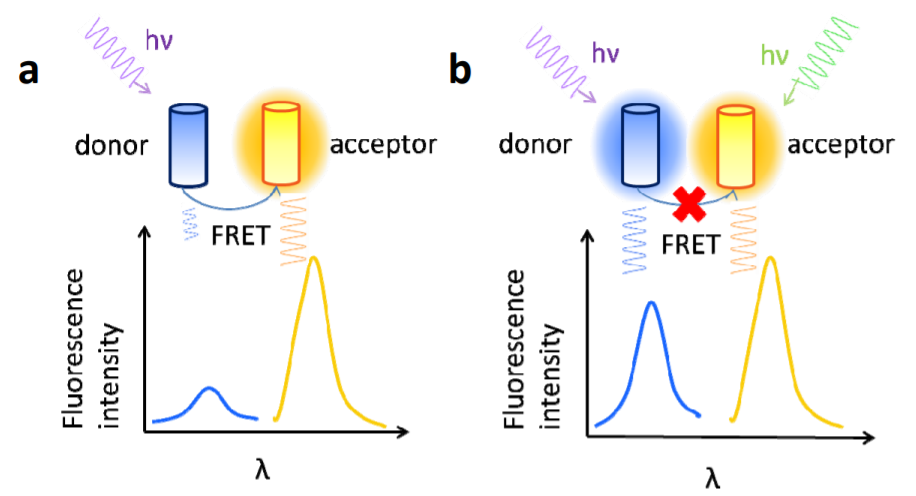
FRET is a phenomenon of energy transfer between two fluorescent molecules which are very close to each other. When the emission spectrum of the donor molecule overlaps with the absorption spectrum of the acceptor molecule, and the distance between the two molecules is within 10nm, a non-radioactive energy transfer, namely FRET, occurs. This makes the fluorescence intensity of the donor much lower than when it exists alone (fluorescence quenching), while the fluorescence emitted by the receptor is greatly enhanced (sensitized fluorescence).
Applications:
- Intercellular interactions
- Study on membrane proteins
- Interaction between cell membrane receptor proteins
- Study on apoptosis
- Nucleic acid test
An ideal FRET interaction system requires a pair of suitable fluorescent substances, that is, the emission spectrum of the donor overlaps with the absorption spectrum of the acceptor. When the excitation wavelength of the donor has no effect on the acceptor, the emission spectra of the donor and acceptor should be completely separated, otherwise it is easy to cause spectral interference and make the reaction system unstable. At present, green fluorescent proteins (GFPs) and dyes are the most commonly used donor receptor pairs. Green fluorescent proteins include CFP-YFP, BFP-GFP, BFP-YFP, and dyes include cy3-cy5, FITC, rhodamine, etc. And these fluorescent substances should be able to be labeled on the research object.
Advantages:
- Under the normal physiological conditions of living cells, the conformation changes and interactions of macromolecules in cells were observed, which made up for the shortcomings of breaking cells to detect interactions;
- It has high sensitivity and can be used to study single cell and single receptor molecule;
- It can be combined with a variety of instruments and technologies, such as microscope, chromatography, electrophoresis, cell loss technology, etc;
If you are interested in our FRET service, Profacgen looks forward to cooperating with you.
Reference:
[1] Fang Xu, Lu Wei, Zhixing Chen, and Wei Min, "Frustrated FRET for high-contrast high-resolution two-photon imaging," Opt. Express 21, 14097-14108 (2013)