Induced Protein Degradation
Tens of thousands of proteins in our cells are always being made and disposed at any given time. This fine balance is mediated by the ubiquitin/proteasome system. The ubiquitin can attach to proteins at using one of its seven lysine residues and can thereby form numerous ubiquitinated products. The type of linkage will largely determine the fate of the tagged proteins—certain linkages will lead to protein degradation. On the other hand, only 20% of the proteins can be accessed by small-molecule drugs and antibody-drug conjugates. To address the conventionally “undruggable” targets, researchers have developed protein degraders (Figure 1) that take advantage of a cell’s natural system for disposing damaged or misfolded proteins, recruiting ubiquitin/proteasome system to break disease-causing proteins.
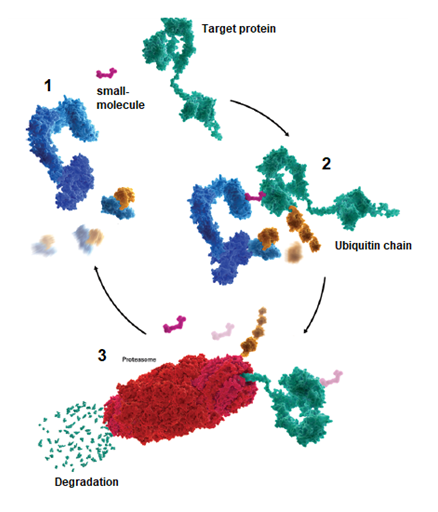 Figure 1. Schematic representation of a small-molecule-mediated protein degradation.
Figure 1. Schematic representation of a small-molecule-mediated protein degradation.
After seeing the early success of protein degraders, the pharmaceutical industry has come to realize that these novel strategies could provide for powerful tools to silence errant proteins with high selectivity and efficacy. Unlike small-molecule drugs, protein degraders could theoretically target any protein of interest, thereby creates myriad therapeutic opportunities.
Profacgen—a state-of-the-art service provider—is dedicated to deliver the most up-to-date biotechnologies, tools and expertise with competitive prices and rapid turnaround time for clients across the globe. The development of induced protein degradation has become an exciting research area with the potential to address unmet medical challenges. Herein, we proudly offer a new set of services that aim to help our clients construct new protein degraders as therapeutic agents and diagnostic tools.
Proteolysis Targeting Chimera (PROTAC)
PROTAC is used to selectively degrade any protein of interest by bringing the target protein into proximity of the E3 ligase for proteasome-mediated proteolysis. The key step is the construction of a heterobifunctional small molecule that has two warheads – one binds to the target protein, and the other recruits the E3 ligase.
Hydrophobic tag has been discovered to enable small-molecule control over the target protein in the absence of a direct ligand, providing an ideal way to study and validate potential drug targets in various disease models.
Destabilization domains (DDs) represent a fusion protein component that is intrinsically unstable and destabilizes other proteins upon incorporation, leading to protein degradation. This approach enables the regulation of secreted proteins and their biological activity, which opens up new avenues for various applications such as cancer therapy and targeted gene delivery.
For more information regarding Profacgen’s induced protein degradation services, please contact us. Our customer service representatives are available 24 hours a day, Monday through Friday, to assist you.