PROTAC
Ubiquitin modification plays an important role in the post-translational modification of proteins. In cells, various E3 ubiquitin ligases catalyze the polyubiquitination of specific proteins, resulting in their degradation in the proteasome, which is the main way proteins are degraded in cells. Proteolysis targeting chimeras, also named PROTAC, is a chemical molecule that contains two different ligands at each end: a ligand that binds the E3 ligase (pink triangle below) and a ligand that binds intracellular proteins (TP: target protein below) (yellow circle below). The two ligands are linked by a linker. Such a chemical molecule can bind both E3 ubiquitin ligase and intracellular proteins, and the targeted protein is polyubiquitinated by recruitment of the targeted protein to the vicinity of E3 ubiquitin ligase (yellow sugar hagg below), which is then degraded by the proteasome (yellow cylinder below). PROTAC can be recycled and is not degraded by proteasomes.
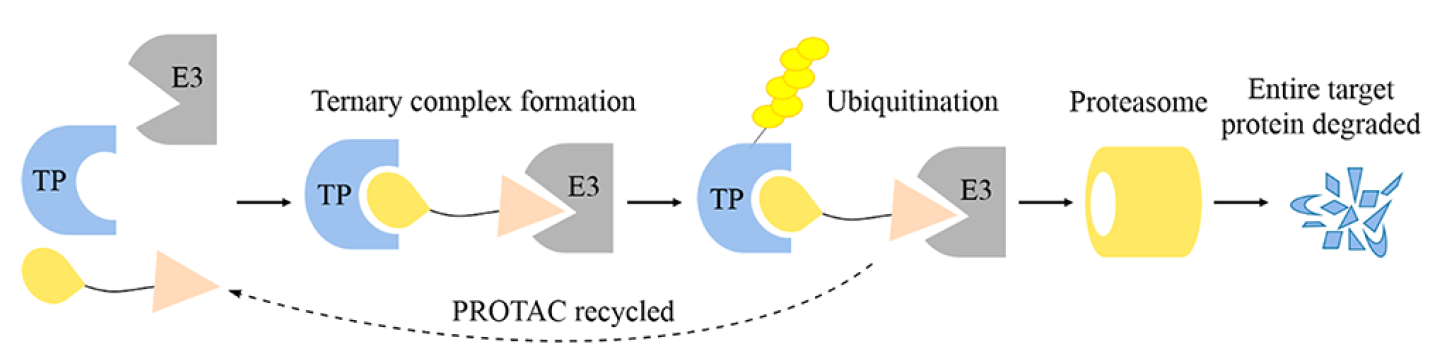
Fig 1 Overview steps of entire target protein degradation by PROTACs [1].
According to the great advantages of PROTAC in drug development, Profacgen protein chemistry department has established the PROTAC technology platform, established the compound library of highly affinity small molecules and small molecule fragments of popular targeted proteins, analyzed a wide range of E3 ligase highly affinity small molecules and small molecule fragments, and designed the PROTAC model of linker system and non-linker system. These accumulated compound libraries can be used for rapid and efficient ligand and targeted protein design, greatly improving the drug development process of PROTAC.
Profacgen provides the following services for you:
E3 ubiquitin ligase, an important component of the ubiquitin proteasome system, is involved in catalyzing ubiquitination of proteins and promoting subsequent protein degradation, thereby affecting a variety of cell physiological activities, including cell cycle, apoptosis, DNA replication, and signal transduction, and is abnormally activated in a variety of tumor cells. Proacgen provides E3 ligase development services, including single subunit E3 ligase, multi-subunit E3 ligase complex, tissue-specific E3 ligase and other target protein development services.
Profacgen can serve the discovery and design of small compound based PROTAC and peptide based PROTAC based ligand. At the same time, the ligand efficiency can be detected and optimized to ensure the physical and chemical properties of the compounds.
Profacgen provide PROTAC in vitro evaluation testing the whole process of comprehensive services, including cytotoxicity test, compounds degradation analysis, stability analysis, E3 ubiquitin ligase activity analysis, and permeability analysis, set up a one-stop testing platform, for the customer to complete PROTAC compounds in all kinds of in vitro testing and assessment services, provide detailed data to meet your individual needs.
In vivo trials refer to experiments within a whole organism, including animal studies and clinical trials. More comparative studies on metabolic, inflammatory, immune, and hormonal pathways are needed to determine the replicability of the results and the optimization of PROTAC. Profacgen provides in vivo evaluation ofa variety of PROTAC candidates.
The targeted protein degradation team at Profacgen is confident in providing targeted protein degradation services for POI based on years of experience in basic and applied research in biochemistry and structural biology.
In view of the great potential of PROTACs in drug development, Profacgen's Protein Chemistry Department has established a powerful PROTAC platform, supporting HaloPROTAC, ATTEC, AUTAC and LYTAC to enhance your drug development process.
Profacgen has an experienced team that provides a variety of PROTAC services. Please contact us for specific project implementation content and time. If you want to know more service information, please feel free to contact us, Profacgen will be your trusted partner.
Reference:
[1] Hongying Gao, Yu Rao. PROTAC Technology: Opportunities and Challenges. ACS Medicinal Chemistry Letters 2020 11 (3), 237-240. DOI: 10.1021/acsmedchemlett.9b00597