A New Method for Detecting RNA-Protein Interaction in Living Cells
As the direct product of genomic DNA, RNA is of vital significance to life activities and is an essential executor of life activities, participating in protein synthesis, post-transcriptional modification and DNA repair, gene expression regulation, and other life processes. Post-transcriptional regulation plays a vital role in biological processes. To a large extent, the underlying molecular mechanism is mediated by the physical interaction of RNA transcripts with a large number of RNA binding proteins (RBPs) that regulate RNA splicing, stability, nuclear export, and translation.
There are various methods to study and characterize interactions between RNA and protein (shown in Fig.1). The research methods can be divided into two categories: detect proteins bound to target RNAs (RNA-centric) and methods that detect RNAs bound to target proteins (protein-centric). RNA-centric assays are generally divided into in vitro and in vivo. In vitro methods are particularly effective for determining which nucleotides and amino acids are involved in known RNA-protein interactions. In vivo approaches are biased towards dynamic studies such as subcellular localization, modifications, or RNA-protein interactions as protein concentrations change. Standard protein-centric screens include CLIP-seq, etc., but they have the defect of poor cross-linking efficiency. Although there are many research methods for RBP, researchers are still constantly improving optimization methods to solve more biological problems.
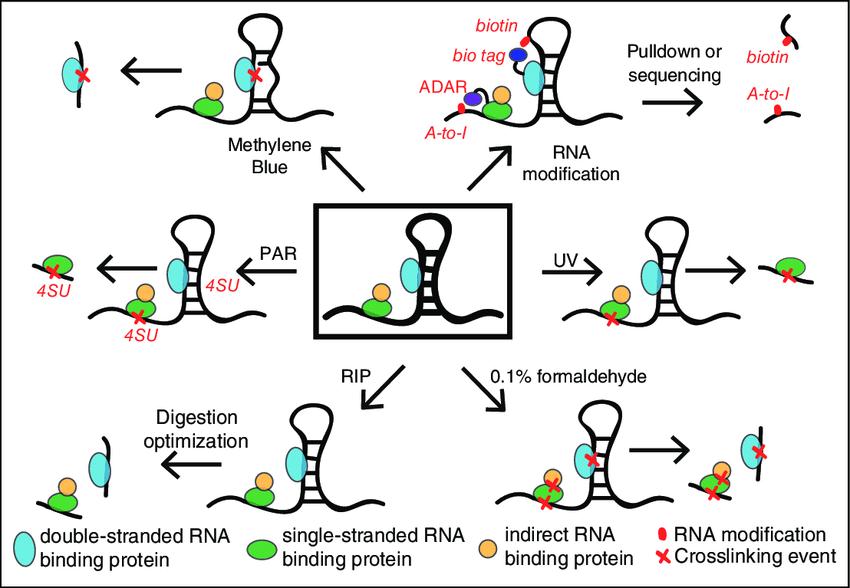 Fig.1 Methods to capture protein–RNA interactions.
Fig.1 Methods to capture protein–RNA interactions.
To overcome the limitations of existing methods, the researchers developed a technique to directly label proteins that bind to specific RNA motifs in living cells. The system utilizes phages to design a tool that uses biotin ligase-tagged proteins to bind to RNA motifs in living cells and detect interactions by streptavidin pull-down assay and mass spectrometry. To achieve the best labeling effect of the system, the researchers optimized the biotinidase used in the system. After analyzing and comparing various biotinidases, the most effective biotin ligase derived from Bacillus subtilis was selected. This method can more rapidly detect the interaction of shorter RNA sequences with proteins while requiring fewer cells and provides a new idea for studying RNA-protein interaction.
The Advantages of this platform include:
- Detecting in living cells
- Experiments can be completed in a short time, and analysis data can be obtained
- Detecting shorter RNA sequences
- Cell samples can be used directly for detection
Potential Applications
- Detection of RNA-Protein binding
- High-throughput detection possible
- Measure reaction kinetics of RNA-Protein binding
Profacgen is a state-of-the-art protein service provider. We provide custom protein services in the biological sciences, enabling access to the latest tools, techniques, and expertise with competitive pricing and rapid turnaround time. We serve a broad spectrum of industrial and academic clients committed to delivering high-quality data and customer services.
Please do not hesitate to contact us for more details if you are interested in this new method, and we will provide a considerate service for you. At the same time, we also offer other RNA-protein interaction detected services; please move to our website for more details.
Reference
- Wheeler, Emily & Van Nostrand, Eric & Yeo, Gene. (2017). Advances and challenges in the detection of transcriptome‐wide protein–RNA interactions. Wiley Interdisciplinary Reviews: RNA. 9. 10.1002/wrna.1436.