Protein ELPylation Service
Elastin like polypeptides (ELPs) are artificial polymers composed of multiple Val-Pro-Gly-Xaa-Gly (VPGXG) pentapeptide repeat units in series. VPGXG comes from the hydrophobic region of mammalian elastin. Xaa is usually Val, Ala, Ile and other amino acids (except Pro). ELPs have phase transition characteristics, that is, when they reach a certain temperature, they can have a reversible transition from solution state to condensed state. This characteristic makes them have a broad application prospect in the fields of biology, medicine, environment and so on.
ELPs belong to one of the three types of heat sensitive peptides, which have the characteristics of changing with the ambient temperature. They are called reversible transition, that is, they are dissolved at a lower standard solution temperature, and change into condensed state when the temperature rises to its phase transition temperature (TT). As the temperature decreases, the dissolved state can be restored. The phase transition mechanism can be clarified from the aspect of conformational change. When the ambient temperature is lower than TT, the freely extended polypeptide chain is fully hydrated and in a disordered state. When the temperature is higher than TT, the disordered polypeptide chain forms a relatively orderly state β-sheet structure. A lamellar structure is further crosslinked and aggregated. When ELPs are fused with other proteins, they can still retain the phase transition characteristics. The parameters affecting TT include the concentration of ELPs, the ionic strength of the solution, the molecular weight of ELPs polypeptide and the polarity of Xaa. The first three parameters are inversely proportional to the value of TT, while the polarity of Xaa is directly related to the phase transition temperature. When Xaa is a hydrophobic group, the phase transition temperature decreases; On the contrary, it increases. ELPs with different TT can be designed according to different polarity of Xaa.
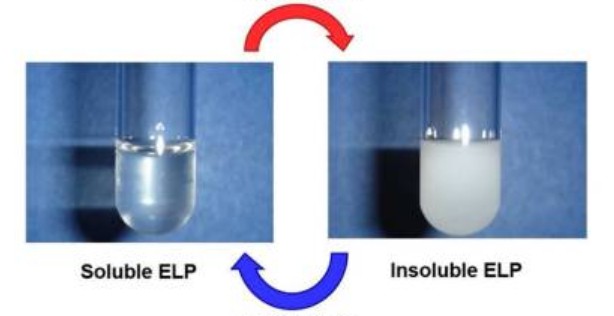 Figure 1. ELPs are soluble below a transition temperature, Tt, but undergo coacervation at temperatures above Tt (Despanie, J.; et al. 2018)
Figure 1. ELPs are soluble below a transition temperature, Tt, but undergo coacervation at temperatures above Tt (Despanie, J.; et al. 2018)
The ideal drug carrier should improve the rate of drug reaching the affected area as much as possible and reduce the toxicity caused by drug absorption by normal tissues. The drug delivery system based on ELPs has two outstanding advantages: first, the molecular weight and composition of ELPs are accurately controllable, which can adjust the drug diffusion rate and cell absorption rate; Secondly, the microparticle structure of ELPs is an amphiphilic block copolymer composed of hydrophilic part and hydrophobic part, which can be self-assembled into hydrophilic shell and hydrophobic core to wrap and carry drugs with poor solubility and stability.
As drug carriers, ELPs mainly carry three types of drugs: (1) small molecule drugs. Paclitaxel is a hot anti-tumor drug in recent years. Because of its low solubility, the formula often contains polyoxyethylene castor oil (Cremophor EL) and ethanol to promote dissolution, which often lead to side effects. The covalent combination of ELP and paclitaxel greatly increased its solubility and solved the problem of poor water solubility to a certain extent. (2) polypeptide or protein drugs. Polypeptide drugs are generally used to regulate the interaction between important proteins in the body. These drugs often cannot pass through the cell membrane. The existence of intracellular peptidase after entering the membrane reduces the stability of these drugs and limits their application. Some polypeptides that inhibit cell cycle, such as KLAK polypeptide (the amino acid sequence is KLAKLAKKLAK), induce apoptosis by interfering with mitochondria. Genetic engineering is used to conjugate KLAK sequence to the C-terminal of ELP, and at the N-terminal to conjugate cell permeable polypeptide sequence SynB1 to increase its cell membrane transmissivity. The fusion peptide formed is toxic to human breast cancer cell lines with negative estrogen receptor. 3) Plasmid and adenovirus vector. Part of the sequence of ELPs and diethylenetriamine modified polyala form a recombinant copolymer P [ASP (DET)] 53elp (1-90). The copolymer retains the phase transition characteristics of ELPs and uses it to transfer pGL2 plasmid. The cytotoxicity is reduced and the transfection rate is greatly improved
The traditional protein expression purification system is to fuse the target protein with a specific carrier protein or polypeptide, and then achieve the purpose of purifying the target protein by means of the specific interaction between the carrier protein or polypeptide and the ligand coupled on the affinity chromatography. However, the protein obtained by this method has low purity and limited column load, so it is not suitable for large-scale industrial production. The non-chromatographic purification method of ELP fusion protein makes up for the above shortcomings. ELPs are fused with the target protein. According to the inverse transition cycling (ITC) process, ELPs fusion protein is aggregated and precipitated by changing the ionic strength and / or temperature of cell lysate. If this is repeated, even if the expression level of fusion protein is in microgram level, it can still achieve high purity. Compared with the traditional label purification method, this method is efficient, fast, and easy to operate without the help of special instruments and equipment. At the same time, the existence of ELPs can also stabilize the fused protein to avoid aggregation and denaturation in high concentration.
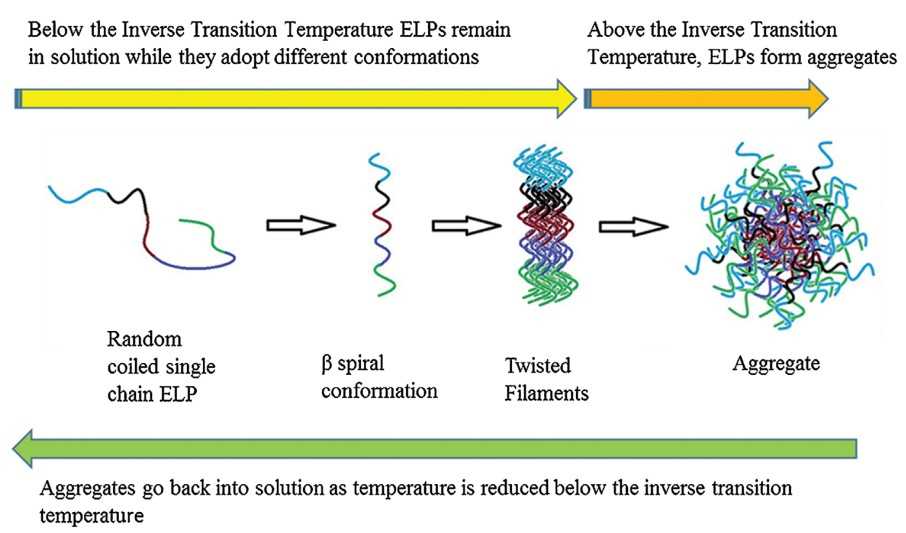 Figure 2. Formation of aggregates by single chain ELPs (Yeboah, A.; et al. 2016)
Figure 2. Formation of aggregates by single chain ELPs (Yeboah, A.; et al. 2016)
Services
Relying on a powerful protein expression and purification platform, Profacgen will provide protein ELPylation services. Our ELPs rely on simple and straightforward purification procedures that can significantly reduce the cost of recombinant protein production. The purification process includes an addition of salt, a warm up step to induce aggregation and precipitation, centrifugation, and protein re-dissolution, etc. Generally, two to three rounds of heating-centrifugation-redissolution steps can purify the desired protein. The ELPylation service we offer eliminates the reliance on expensive chromatographic equipment, resins and reagents and thus represents a very cost-effective and easily scalable purification process.
Our services include but are not limited to:
- In addition to the E. coli ELP fusion protein expression system, the CHO expression system is also available
- Assessing post-translational modifications of fusion proteins
- Provide multiple continuous flow ultracentrifuges with multiple bioreactors, and continuous flow membrane filtration system
- Provides TEV and thrombin recognizable sequences as fusion linkers, as well as self-cleaving
Profacgen has accumulated lots of experience in protein labeling. Our professional technical team can provide customers with high-quality protein ELPylation and many related featured services. Our competitive prices and extensive expertise have earned us the trust of our collaborators. Contact us to find out how Profacgen could be of assistance.
References
- Yeboah, A.; et al. Elastin-like polypeptides: A strategic fusion partner for biologics. Biotechnology and Bioengineering. 2016.
- Despanie, J.; et al. Elastin-Like Polypeptides: Therapeutic Applications for an Emerging Class of Nanomedicines. J Control Release. 2018.