Bimolecular fluorescence complementation (BiFC) technology refers to two proteins with interaction affinity, which relate to each other to form a complete fluorescent protein, thus characterizing the occurrence and spatial location of protein-protein interaction.
BiFC is a new technology first reported by Hu et al., which can directly and quickly determine the location and interaction of target proteins in living cells. It has been reported that there are many specific heterotopic spots on the loop structure between two lamellae of GFP, which can insert foreign proteins without affecting the fluorescence activity of GFP. BiFC technology takes advantage of this characteristic of the fluorescent protein family, The fluorescent protein was divided into two non-fluorescent fragments, and then connected with the target protein respectively. If the two target proteins are close to each other due to interaction, the two molecular fragments of the fluorescent protein will be close to each other in space, forming active fluorescent gene and emitting fluorescence. Under the fluorescence microscope, we can directly observe whether the two target proteins interact with each other, and observe the time, location, strength, stability of the formed protein complex and the influence of cell signal molecules on the interaction under the condition closest to the physiological state of living cells. This information is of great significance to the study of protein-protein interaction.
Many methods have been developed to detect biomolecular interactions, but most of them rely on sophisticated instruments and complex data processing. BiFC technology can simply and directly observe the interaction events in living cells. This method has been used successfully in many different cell types and organisms and does not require structural information of the interaction partners and exogenous fluorophores or dyes to stain the cells.
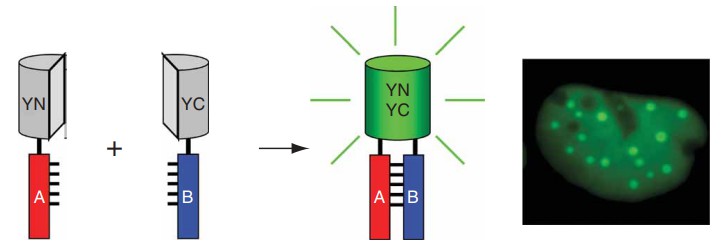 Figure 1. Schematic diagram representing the principle of the BiFC assay (Kerppola, T.K. 2006)
Figure 1. Schematic diagram representing the principle of the BiFC assay (Kerppola, T.K. 2006)
The system is suitable for the study of protein-protein interaction with strong protein-protein interaction. The fluorescence intensity of YFP is controlled at a low level and is not interfered by background and other factors.
The system is suitable for the study of protein-protein interaction with weak interaction, such as membrane protein interaction. YFPn and YFPc are edited manually, and YFP fluorescence is enhanced, which is convenient for observing the weak interaction signal.
The system can be used to observe and take pictures of living system, especially for quantitative analysis and comparison of interaction strength. It is suitable for the study of protein interaction influenced by third-party substances. For example, the strength of protein interaction can be regulated by hormones and small molecular compounds, and the change of interaction strength can be determined by the observation of exogenous hormone compounds.
As a protein research expert, Profacgen has accumulated a lot of successful experience in protein interaction analysis, and has assisted many customers around the world to complete projects with high efficiency and high quality. Our highly competitive prices and unprecedented expertise have earned us widespread acclaim. Contact us to find out how Profacgen could be of assistance.
References
Fill out this form and one of our experts will respond to you within one business day.