Recombinant Protein Expression in Insect Cells Using the Baculovirus Expression System
Background
Recombinant baculovirus are widely used in the expression of heterologous genes in cultured insect cells. Baculovirus is a family of DNA viruses. They are one of the most prominent viruses known to affect the insect population[1]. Generally, the process of viral replication is divided into three phases, throughout which wild-type baculovirus exhibits both lytic and occluded life cycles. The three phases are characterized as follows:
| 1. | Early Phase: In this phase, the virus infects the insect cell by attachment, penetration and uncoating. In this phase, the infected cells are prepared for viral DNA replication. Normally, initial viral synthesis occurs 0.5-6h post-infection, along with the shutting down of host gene expression. |
| 2. | Late Phase: Genes that code for replication of viral DNA and assembly of virus are expressed during this time. Cells begin to produce extra-cellular virus that contains the plasma membrane envelope and glycoprotein during the time range of 6-12h post-infection. Both are necessary elements for viral infection through the process of endocytosis. The virions are then assembled and budded. Recombinant virions are released 18-36h post-infection. |
| 3. | Very Late Phase: Occlusion derived virus particles are produced and cell lysis occurs in this phase. |
Accordingly, several morphological changes of the infected cells can be observed as virus infection advances. The time points of the infection cycle and the cellular morphological changes vary with the insect cell line and baculovirus strain used. However, there are several common behaviors presented by the infected cells [2].
| 1. | Early Phase: The virus nucleocapsids pass through the cytoplasm to the nucleus. With the release of the contents of the capsids, the cellular structure changes within the early hours following infection. Normal cellular functions decline sharply. |
| 2. | Late Phase: Most of the cellular functions cease within 6-24h post-infection. The infected cells stop dividing; the production of viral genome and budded virus increases. Increased cell diameter and enlarged nuclei can also be observed. |
| 3. | Very Late Phase: The infected cells cease production of budded virus and initiate the assembly, production and expression of the recombinant protein within 20-36h post-infection. The density of cell culture decreases dramatically as cells die and lyse. The infected cells continue to increase in diameter and display enlarged nuclei. Vacuoles are visible among the cytoplasm and the nuclei may demonstrate granularity to some extent. |
Advantages of BEVS Technology
Recombinant baculovirusis widely used in the expression of heterologous genes in cultured insect cells. The baculovirus expression vector system (BEVS) is particularly advantageous in large-scale applications. It utilizes efficient site-specific transposition system to generate recombinant baculovirus for high-level expression of recombinant proteins. When used as expression vectors, the inserted heterologous genes are placed under the transcriptional control of a strong promoter, which ensures the expression of the target protein. The expressed recombinant proteins are then processed, modified and targeted to the appropriate cellular locations. There are certain essential elements involved in the Baculovirus expression system that contribute to a rapid and efficient method to generate recombinant baculoviruses.
| Components | Functions |
|---|---|
| ☆ A Bac donor plasmid | • Allow generation of an expression construct containing the gene of interest; • A baculovirus-specific promoter controls its expression. |
| ☆ An E.coli host strain | • Contain a baculovirus shuttle vector and a helper plasmid; • Allow generation of a recombinant baculovirus shuttle vector following transposition of the target gene to the Bac expression construct. |
| ☆ A control expression plasmid | • Contain the Gus/CAT gene that allows production of a recombinant baculovirus; • Express β-glucuronidase/chloramphenicol acetyl-transferase when used to infect insect cells. |
| ☆ Evaluation of culturing conditions | Chosen insect cell lines, growth media (with/without serum) and feeding/infection strategies that allow for optimal product expression. |
Our trained custom services group will perform all the steps for the fastest expression of your target protein using the Baculovirus Expression System, which saves your resources and time.
Experimental Outline
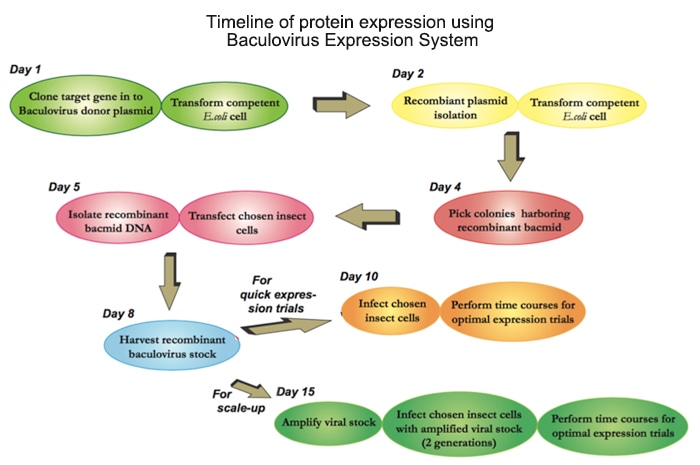
The flow chart below illustrates the general steps required to express your target protein using the Baculovirus Expression System.
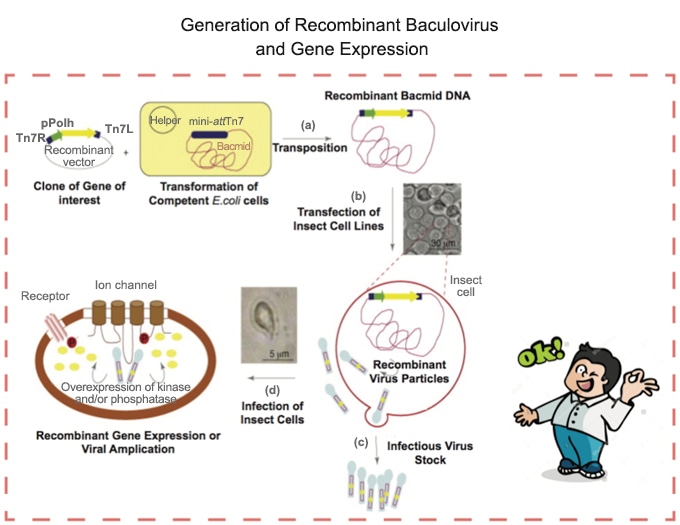
Protocol for Baculovirus Preparation
You can find below what we normally do in our lab to use the baculovirus system:
1. Culturing of insect cells.
Insect cells can be very sensitive to environmental factors. Therefore, some chemical, nutritional and physical culture factors (temperature, medium osmolality, aeration, pH and shear forces) need to be optimized before culturing insect cells.
2. Cloning into Baculovirus donor plasmid.
Insert the target gene in frame with the Baculovirus donor plasmid in which the target protein is fused to a 6*Histidine tag and a protease cleavage site. The cloned gene can be inserted downstream/upstream of the tag for fusion protein expression, which depends on the expression condition and stability of your target protein. The protease cleavage site between the protein tag and your target protein will allow the removal of the tag from the target protein after purification. In addition, the vector also contains antibiotic resistance gene for further clone selection. We can also design the plasmid according to your specific needs.
3. Transformation & analysis.
Once we have cloned the target gene into the Baculovirus donor vector, chemical transformation of the ligation product into E.coli strain is performed for recombinant plasmid screening.
| i. | Add the target vector into thawed competent cell. |
| ii. | Incubate the cells on ice, and then treat with heat-shock and ice-shock. |
| iii. | Add liquid medium for the treated competent cells to recover. |
| iv. | Plate out the cells onto agar plates containing corresponding antibiotic for transformant selection. |
| v. | Isolate recombinant plasmid DNA and analyze by restriction enzyme digestion to further confirm the correct orientation of the inserted DNA. Alternatively, the positive transformants can be verified by PCR method using appropriate forward and reverse PCR primers. |
| vi. | DNA sequencing result will be used as a final confirmation of the correct insertion of your target gene. |
| vii. | Prepare a glycerol stock of the purified correct clone for long-term storage. |
4. Generation of Bacmid.
In this step, the constructed target gene-Baculovirus donor vector will transform a special E.coli strain for transformation into the bacmid. Blue-white selection will be performed to identify colonies containing the recombinant bacmid.
| i. | Add the plasmid DNA into thawed competent cell. |
| ii. | Incubate cells on ice, and then treat with heat-shock and ice-shock successively. |
| iii. | Add liquid media (at room temperature) for the treated competent cells to recover. |
| iv. | Plate the cells onto agar plates containing antibiotics for transformant selection. |
| v. | Select white colonies for analysis. It normally takes 24 hours before white and blue colonies can be distinguished by naked eyes. |
| vi. | Pick white colonies and restreak them with fresh LB agar plates containing antibiotics plus Bluo-gal and IPTG. |
| vii. | Choose a single white colony on the restreaked plates and inoculate a liquid LB culture containing antibiotics. |
| viii. | Isolate recombinant bacmid DNA. |
| ix. | Perform PCR analysis of the recombinant bacmid DNA followed by agarose gel electrophoresis to verify the successful transposition to the bacmid. |
Note that the amplification result of your recombinant bacmid DNA could vary a lot depending on different combinations of PCR Forward and Reverse primers you choose. Calculate the expected size of your PCR product before analyzing the amplified DNA bands.
5. Produce the 1st generation of Recombinant Baculovirus.
Once we confirm the presence and correct orientation of the target gene within the recombinant bacmid, the next step will be the transfection of insect cell to produce the first batch of recombinant baculovirus.
| i. | Make sure that the purified recombinant Bacmid DNA for transfection be free of phenol and NaCl. Contaminants may kill the insect cells, and salt may decrease transfection efficiency by interfering with lipid complexion. |
| ii. | Calculate the number of insect cells for transfection experiment. It’s of great importance that the cells should be healthy and up to 97% viability prior to transfection. |
| iii. | Dilute the purified bacmid DNA and transfection reagent with growth media respectively. |
| iv. | Gently mix the diluted bacmid DNA with the Transfection reagent at a series of combination ratios. Incubate at room temperature before use. |
| v. | Add the gently mixed DNA: lipid complex into the insect cells. |
| vi. | Remove the complexes from cell culture after incubation. |
| vii. | Add fresh growth medium to the cells. |
| viii. | Incubate the cells for at least 3 days until it shows certain signs of viral infection in the cell culture. |
Note that the highest transfection efficiency maybe reached by optimizing transfection conditions, such as altering DNA/Transfection reagent concentrations/ratios, and adjusting cell density.
6. Isolating the 1st passage (P1) of baculoviral stock.
Amplified virus should be released into the medium several days post transfection depending on transfection efficiency. Once the cells are visually inspected to display signs of infection, the virus should be harvested from cell culture medium as following steps:
| i. | Collect the medium and transfer to centrifuge tubes. |
| ii. | Centrifuge to remove cells and large debris. |
| iii. | Transfer the supernatant to a new centrifuge tube. |
| iv. | Store the 1st passage (P1) viral stock at 4°C. |
Note that for long-term storage, store an aliquot of the viral stock at -80°C for future re-amplification. Avoid repeated freeze-thaw cycles.
7. Amplify P1 baculoviral stock (optional).
Since the P1 viral stock is a low-titer stock, we can amplify the recombinant baculovirus to prepare a second passage (P2) of viral stock for later expression experiments.
| i. | Prepare insect cell suspension and allow them to reattach prior to infection. |
| ii. | Add the calculated amount of P1 viral stock. |
| iii. | Incubate the cells and harvest the virus several days after infection followed the procedure in Step 6. |
| iv. | Store the P2 viral stock at 4°C or -80°C for long-term storage. |
Note that the optimal harvest times can vary among each baculoviral construct. We can also scale-up the amplification procedure to the volume you desire following the step described above.
8. Preliminary expression experiments.
Please refer to the general guidelines provided below to see how to infect cells using the recombinant baculovirus for the expression of a target protein [5].
| i. | Prepare insect cell suspension and allow them to reattach prior to infection. |
| ii. | Remove the medium and rinse the cells with fresh media. |
| iii. | Add there combinant baculovirus into the cell culture and continue to incubate the cells. |
| iv. | Harvest cells or media (depending on the expression pattern of the recombinant protein) at different time points. |
| v. | For recombinant protein expressed within insect cells, remove the medium and lyse cells with appropriate lysis buffer following rinse. |
| vi. | Freeze and boil the samples before SDS-PAGE or western blot analysis. |
Click here to contact us for more technical information.
References:
[1]Summers M D, Anderson D L. Characterization of nuclear polyhedrosis virus DNAs[J]. J Virol, 1973, 12(6): 1336-46.
[2]Luckow V A, Lee S C, Barry G F, et al. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli[J]. J Virol, 1993, 67(8): 4566-79.
[3]Ciccarone V C, Polayes D A, Luckow V A. Generation of Recombinant Baculovirus DNA in E.coli Using a Baculovirus Shuttle Vector[J]. Methods Mol Med, 1998, 13: 213-35.
[4]Luckow V A, Summers M D. Signals important for high-level expression of foreign genes in Autographa californica nuclear polyhedrosis virus expression vectors[J]. Virology, 1988, 167(1): 56-71.
[5]Lahtinen T, Linder M B, Nakari-Setala T, et al. Hydrophobin (HFBI): A potential fusion partner for one-step purification of recombinant proteins from insect cells[J]. Protein Expr Purif, 2008, 59(1): 18-24.