Recombinant Protein Expression in Mammalian Cells (HEK293/CHO)
Introduction to Expression in Mammalian Cells
Many proteins can be expressed at high level in Escherichia coli. However, prokaryotic expression systems fail to generate correctly folded functional forms of many eukaryotic proteins, and hence a variety of eukaryotic-based expression systems have been developed [1].
Mammalian cell expression systems are the ideal choice to produce eukaryotic proteins as they enable post-translational modifications which are crucial for the correct folding of many eukaryotic proteins. Secreted mammalian proteins have been successfully produced in a large-scale format in Chinese hamster ovary (CHO) cells [2] and human embryonic kidney (HEK) 293 cells [3-5]. Glycoproteins are also generally synthesized in mammalian cells, because common microbial hosts like E.coli lack the requisite machinery to synthesize appropriate glycoforms. Despite the availability of a plentitude of cell lines, nearly 70% of all recombinant protein therapeutics produced today are made in CHO cells [7].
Mammalian cell expression systems have been traditionally seen as expensive due to the cost associated with transfection reagents, specialized media, consumables and the expert workforce required [1]. However, the cost of PEI reagent per litre of transfected cells is very close to zero. HEK293T cells are very easy to maintain and they grow in standard DMEM, one of the cheapest eukaryotic cell media available. The costs associated with plastic ware have already been reduced by the use of expanded-surface roller bottle. Proper training in the use of sterile techniques and good technical support are indeed crucial, but these are skills worth investment.
Here we present our cost-effective and fast mammalian cell-based expression system that will not only support small-scale screening of a large number of constructs in parallel, but will also allow rapid and reliable scale-up production for milligram-quantity recombinant proteins.
Experimental Outline
Flow Chart of the Transient Mammalian Expression System
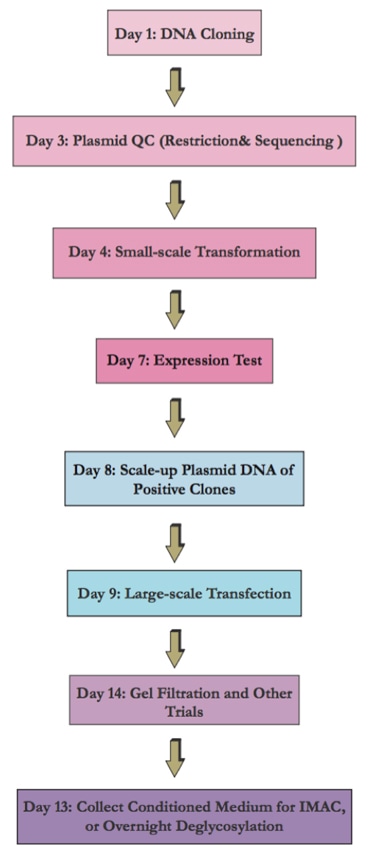
Flow Chart of the Stable Expression Cell Line Development
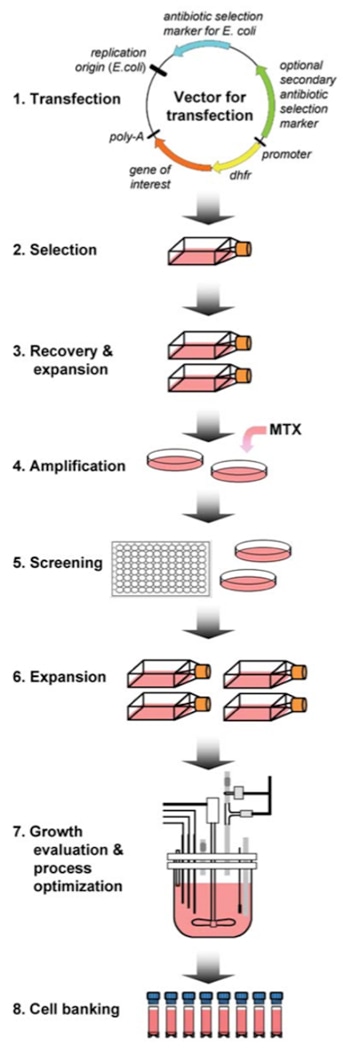
Protocol for Recombinant Protein Expression using the Mammalian Expression System
The following protocol can be a reference for both transient and stable gene expression using HEK293 cells.
1. Plasmid preparation
| a) | Prepare pure plasmids for cell transfection could i. Maximize transfection efficiency ii. Minimize cell death from endotoxin |
| b) | Prepare 250 mL bacteria culture. |
| c) | Purify with Endo-free Maxi Prep Kit or use cesium chloride purification instead. |
2. Linearization of the expression plasmid
| a) | Recommended for stable cell lines. |
| b) | Choose restriction site in non-essential location. |
| Please note that the selected location SHOULD NOT be within or close to genes that are to be expressed. | |
| c) | Chances are that integration doesn’t occur in your inserted gene, which is the most common reason for growth of resistant cells that don’t express your inserted gene. |
3. Transfection
Optimal transfection is achieved when adherent cells reach about 90% confluency.
| a) | For a standard 15 cm tissue-culture dish (approximate surface area 176 cm2), 50 µg of plasmid DNA is required (quantities need to be scaled accordingly for the different surface areas of plates/flasks/bottles). |
| b) | Mix DNA with PEI stock (1 mg/ml). Vortex briefly . |
| c) | Incubate the solution at room temperature to allow DNA–PEI complex formation. We found that the optimal DNA:PEI ratio is between 1:1.5 and 1:2; however, we prefer 1:1.5 owing to lower toxicity. |
| d) | During complex formation, change the media from the plates to be transfected, lower the serum concentration to 2%. |
| e) | Finally, the DNA–PEI complex is added to the dish, which is then briefly rotated to allow mixing. |
| f) | Place cells in the incubator. |
In this section, positive control will be included to monitor transfection efficiency.
4. Evaluation of the transfection efficiency
| a) | 2 days post transfection. |
| b) | Counting the percentage of GFP-positive cells i. Commonly 10-80% |
| c) | Or, quantitate mRNA expression of the inserted gene by RT-PCR. This will require harvesting at least 10% of cells. |
5. Harvest cells for experiment.
Besides, 10% of cells can be returned to flask for stable cell line generation (next).
6. Generate stable cell line
| a) | Add media with selection agent 48 hours post transfection. |
| b) | Allow up to 2 weeks for selection and growth of resistant clones. Refresh media when old media starts to turn yellow. |
| c) | Transfer visible clonal colonies to a 6-well plate. i. Collect at least 6-12 colonies ii. Change to media with antibiotics (Pen/Strep and half strength selection antibiotic, 400 ug/mL Geneticin) immediately before picking up colonies. |
| d) | Allow to reach about 80% confluency and maintain concentration of antibiotics. |
| e) | Harvest 90% of cells and evaluate for expression of the inserted gene. i. RT-PCR (best for gene sequence variants) ii. Activity for enzyme iii. Western blot if appropriate antibody is available. |
| f) | Transfer the best clones to T-75 for scale-up production. |
7. Purification
| a) | Recombinant proteins are readily purified from conditioned media, with protocols that vary depending on the affinity tags used. |
| b) | We normally purify His-tagged constructs from large-scale cultures using the following IMAC batch procedure. Conditioned media are filtered through a 0.2 µm membrane, diluted three fold with buffer solution and adjusted to final pH by adding Tris buffer. |
| c) | IMAC purification is performed using Nickel-coated chelating Sepharose. |
| d) | A final round of gel filtration is usually sufficient to yield proteins of over 90% purity. |
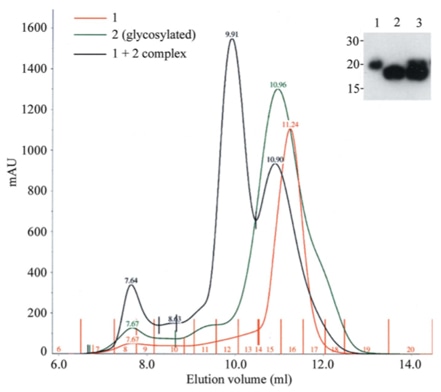 |
The above figure reveals the efficient production of a receptor-ligand complex by co-transfection in HEK293T cells. Cells were transfected with either the receptor construct (sample 1) or the ligand (sample 2, N-glycosylated) or a 1:1 mix of the two plasmids (1 + 2 complex and lane 3 in the inset) [1].
Click here to contact us for more technical information.
References:
[1] Aricescu A R, Lu W, Jones E Y. A time- and cost-efficient system for high-level protein production in mammalian cells[J]. Acta Crystallogr D Biol Crystallogr, 2006, 62(Pt 10): 1243-50.
[2] Cockett M I, Bebbington C R, Yarranton G T. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification[J]. Biotechnology (N Y), 1990, 8(7): 662-7.
[3] Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells[J]. Nucleic Acids Res, 2002, 30(2): E9.
[4] Meissner P, Pick H, Kulangara A, et al. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells[J]. Biotechnol Bioeng, 2001, 75(2): 197-203.
[5] Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production[J]. J Struct Funct Genomics, 2005, 6(2-3): 165-70.
[6] Mancia F, Patel S D, Rajala M W, et al. Optimization of protein production in mammalian cells with a coexpressed fluorescent marker[J]. Structure, 2004, 12(8): 1355-60.
[7] Jayapal K P, Wlaschin K F, Hu W-S. Recombinant protein therapeutics from CHO cells - 20 years and counting.[J]. CHO consortium SBE special section.