Biosimilar Testing Services
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product. Biosimilars are officially approved versions of original "innovator" products, and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biosimilars generally exhibit high molecular complexity, and may be quite sensitive to changes in manufacturing processes. That is why you need accurate analysis at every stage.
A biosimilar marketing application should be submitted under the 351(k) section of the Public Health Service (PHS) act. To prove biosimilarity, such an application must include information about analytical studies, animal data, and clinical studies. According to section 351(k) of the PHS act, analytical testings should "demonstrate that the biological product is highly similar to the reference product notwithstanding minor differences in clinically inactive components." Understandably, analytical testings should be a crucial first step in a biosimilar development program.
Biosimilar Development Pipeline
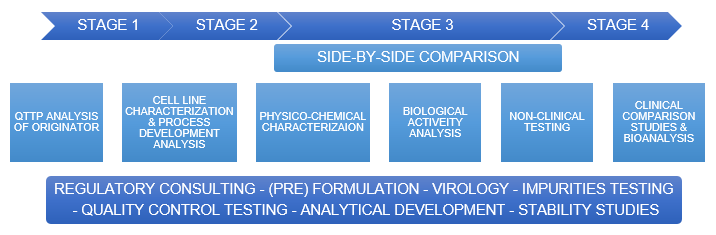
Profacgen provides a one-stop solution for biosimilar testing, including Toxicology, TK and Immunogenicity assessment. We have developed more than a dozen biologics/ biosimilar for toxicology studies. Ranging from determining the exact sequence, structure and quality attributes of the originator to comparative head-to-head biosimilarity testing of the biosimilar with the originator, our biosimilar testing services provide vital analytics.
Profacgen offers following Biologics/Biosimilar services.
| Protein Physico-chemical Properties Molecular mass: Ultracentrifugation, SDS-PAGE, SE-HPLC, Laser light scattering; Isoforms: Isoelectric focusing, Capillary electrophoresis, IE-HPLC; Extinction coefficient determination and validation. |
|
| Protein Structure Analyses Primary structure: Edman degradation, Peptide mapping with LC-MS, C-terminal sequencing, Amino acid analysis; Secondary structure: Peptide mapping with reduced/non-reduced hydrolysis and Edman degradation or MS analysis to show disulphide bonding and other structural forms, Near Ultraviolet Circular Dichroism; Tertiary and Quaternary structure: Far Ultraviolet Circular Dichroism, NMR, FTIR, X-Ray crystallography; Free thiol determination; Carbohydrate structure: Including enzymatic glycan cleavage and MALDI-MS, chromatography; Glycosylation detection/fingerprinting; Characterization of Post Translational Modifications; Crystal structure with microscopy. |
|
| Biological activities In vivo activity: measuring therapeutic effect in animals; In vitro activity: measuring therapeutic effect in cells: cell proliferation, inhibition of proliferation, cell senescence, measurable changes in cell size or content; Enzyme assays; Receptor-binding assays; Promotion or inhibition of coagulation: chromogenic or turbidimetric techniques. |
Our integrated biosimilar development services are based on a step-by-step approach to help you move seamlessly through the critical stages of development – from product characterization and biosafety testing, to full comparability studies and clinical trials, including bioanalytical and bioassay. We customize the service according to the specific requirements from the customers. Please do not hesitate to contact us for more details about our membrane protein modeling service.