Enzyme fragment complementary analysis is derived from the protein-protein interaction (PPI) method[1]. This synergy between this methodology and genetics of complex traits opens up new avenues for studying the molecular etiology of human diseases and promoting their prevention or treatment. Profacgen has developed an enzyme fragment complementation analysis system based on reporter proteins such as β-galactosidase, luciferase, and dihydrofolate reductase.
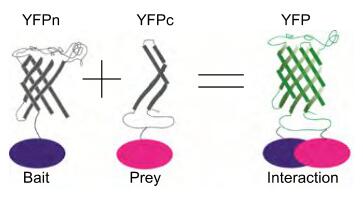
Figure 1. Enzyme Fragment Complementation Assay [1].
Studies showed that after dividing certain proteins into two fragments, both fragments are inactive and lose the function of the protein. But when they are close enough, specific non-covalent complementation occurs and reassembles into a complete protein, thereby restoring protein activity. Based on this, we have established a β-galactosidase-based enzyme fragment complementation system in bacteria. The specific reporter protein β-galactosidase was selected, and it was reasonably divided into two fragments, named the enzyme donor (ED, small fragment) and the enzyme acceptor (EA, large fragment). The target proteins X and Y, when X can interact with Y, the two fusion fragments are complementary and reassembled, thereby restoring the natural structure and function of the reporter protein β-galactosidase. Finally, we use flow cytometry or the luciferase reporting system to detect whether X and Y interact, or to quantitatively determine β-galactosidase activity by ONPG hydrolysis method, so as to detect the protein interaction.
Protocal of Enzyme Fragment Complementation Assay:
Proteins in pathways that interact with X can be further verified by cellular immunofluorescence experiments.
Advantages of Enzyme Fragment Complementation Assay:
Profacgen provides a high-throughput drug screening method based on EFCA and can choose different functional proteins for EFCA, such as luciferase and fluorescent protein, according to the needs of different experiments. We take drug targets as the main object to capture the dynamic changes in the strength and location of intracellular protein interactions, and reflect the activation or inhibition of various cellular pathways (like Toll-like receptor pathway,etc.) by drugs.Thus, the biological activity of the drug is exhibited to the greatest extent.
[1] Zhu, J., Songyang, Z. (2018). Phosphorylation of PLIN3 by AMPK promotes dispersion of lipid droplets during starvation. Protein & Cell. doi:10.1007/s13238-018-0593-9
Fill out this form and one of our experts will respond to you within one business day.