Our labeling strategies facilitate the covalent attachment of various molecules, including biotin, fluorophores (R-PE/APC), reporter enzymes, and radioactive isotopes, to the target protein or peptide. Choice of label depends on the specific application. In other words, the type of label and the labeling strategy should be tailored for each application. Here we take allophycocyanin (APC) labeling as an example to introduce one of our common workflows for protein labeling.
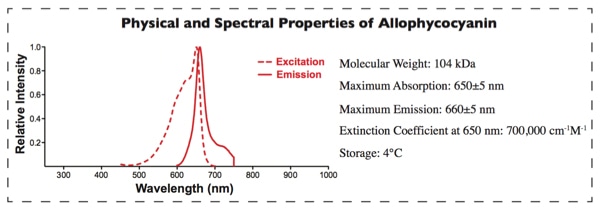
APC Labeling provides a fast way to conjugate proteins/antibodies to APC. APC belongs to the phycobiliproteins family of highly soluble and fluorescent proteins derived from cyanobacteria and eukaryotic algae [1,2]. APC, an ultra-sensitive fluorescent tracer with high emission quantum yields, emits in the near-infrared fluorescence range. It avoids the interference from autofluorescence of cellular components and other biological materials, which made it more sensitive than conventional organic fluorophores. Therefore, it is used in a wide range of applications such as flow cytometry, immunoassays and FRET assay [3,4].
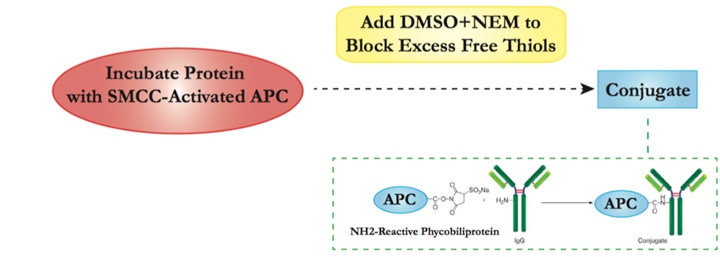
Our APC Labeling strategy is based on the SH-reactive labeling method. The maleimide groups on Succinimidyl 4- c(N—maleimidomethyl) cyclohexane-1-1carboxylate (SMCC) modified APC can easily react with SH groups in the target protein without the need for additional activation. This way, APC can be covalently conjugated to the target protein or antibody in a very simple process.
The following protocol can be a reference for you on APC labeling.
1. Target Protein Preparation
| 1.1 | Adjust protein concentration. | ||||
|
|||||
| 1.2 | Add DTT solution and Mix the reagents gently. | ||||
| 1.3 | Incubate the reaction solution in a tightly capped tube at room temperature without agitation. | ||||
| 1.4 | Desalt the reduced antibody. | ||||
| 1.5 | Calculate the eluted protein concentration according to absorption at 280 nm. It is necessary to keep the antibody solution with a concentration of above 2 mg/mL for better yield in the conjugation reaction. |
2. Conjugation
| 2.1 | Add SMCC-activated APC into target protein solution. Incubate at room temperature with agitation and protect from light. |
| 2.2 | Add DMSO into N-ethyl maleimide (NEM) before mixing with conjugation solution from step 2.1 to block excess free thiols. |
| 2.3 | Once conjugation is completed, the reaction mixture should mainly contain the APC-protein conjugate, with small amounts of free APC and protein. Unconjugated APC should not interfere with the assay. Further affinity column purification can be performed if higher purity is required. |
| 2.4 | The APC conjugation efficiency can be calculated through an formula based on absorbance at 280 nm (protein Amax) and 650 (APC Amax). |
3. Storage of Conjugates
| 3.1 | The APC-labeled conjugate is stored at a concentration above 0.5 mg/mL. BSA (1-10 mg/mL) can be added as a stabilizer. |
| 3.2 | Add preservative (e.g. 0.01% sodium azide). |
| 3.3 | Store at 4°C for no longer than 2 months. Avoid light. |
| 3.4 | Centrifuge briefly and use the supernatant only if aggregation forms. |
Please note that our labeling technique described above is an in-vitro method that is based on purified proteins only.
[1] Brejc K, Ficner R, Huber R, et al. Isolation, crystallization, crystal structure analysis and refinement of allophycocyanin from the cyanobacterium Spirulina platensis at 2.3 A resolution[J]. J Mol Biol, 1995, 249(2): 424-40.
[2] Sidler W, Gysi J, Isker E, et al. The complete amino acid sequence of both subunits of allophycocyanin, a light harvesting protein-pigment complex from the cyanobacterium Mastigocladus laminosus[J]. Hoppe Seylers Z Physiol Chem, 1981, 362(6): 611-28.
[3] Kronick M N, Grossman P D. Immunoassay techniques with fluorescent phycobiliprotein conjugates[J]. Clin Chem, 1983, 29(9): 1582-6.
[4] Oi V T, Glazer A N, Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules[J]. J Cell Biol, 1982, 93(3): 981-6.
Fill out this form and one of our experts will respond to you within one business day.