Circular dichroism (CD) is an absorption spectroscopy method based on the differential absorption of left and right circularly polarized light, which is used to measure the difference in the absorbance of substances to right and left circularly polarized light. In the regular secondary structure of a protein or peptide, the peptide bonds are highly regularly arranged. Proteins or polypeptides with different secondary structures will produce CD bands with different positions and absorption strengths. Therefore, the secondary structure information of the protein or polypeptide chain can be obtained according to the CD spectrum, thereby revealing the structure of the protein or polypeptide.
Currently, this technique has been used to determine various aspects of protein secondary structure, including determining whether an expressed, purified protein is folded, or whether conditions such as mutation affect its conformation or stability. In addition, it is also widely used to study the interaction of proteins with various types of molecules.
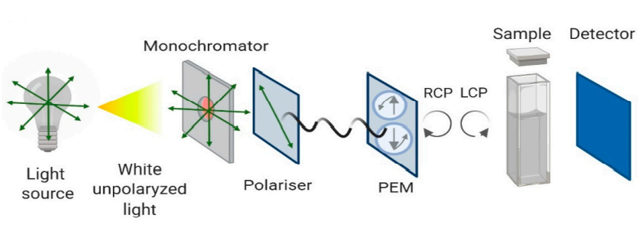 Figure 1. Schematic representation of the Circular Dichroism instrument configuration (Pignataro, M.F.; et al. 2020)
Figure 1. Schematic representation of the Circular Dichroism instrument configuration (Pignataro, M.F.; et al. 2020)
As a protein research expert, Profacgen introduced CD spectroscopy technology to provide customers with comprehensive protein analysis services. We have optimized for both far-UV and near-UV CD for deeper exploration of the tertiary and secondary structures of protein-based biomolecules. In this process, information on stability, folding and unfolding, as well as CD fingerprints and proportion of α-helix and β-sheet of the secondary structure of biomolecules can be provided. In addition to this, CD fingerprints of the tertiary structure of biomolecules obtained in the near UV range (>250 nm) provide usable information on the molecular 3D structure.
Our one-stop circular dichroism service enables efficient, high-quality analysis of structures and interactions ranging from small chiral drugs and transition metal complexes to biological macromolecules.
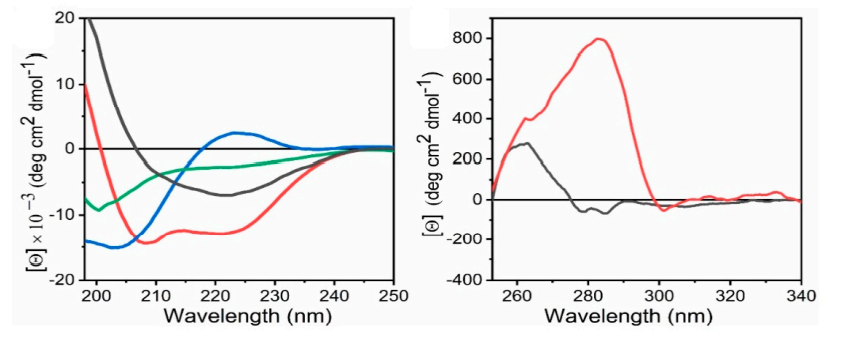 Figure 2. Representative of far-UV CD spectra and near-UV CD spectra (Pignataro, M.F.; et al. 2020)
Figure 2. Representative of far-UV CD spectra and near-UV CD spectra (Pignataro, M.F.; et al. 2020)
Multiple spectrum facilities
Optimized techniques and algorithms
Rapid turnaround time
High accuracy and cost-effective
One-stop and customized service
As a protein research expert, Profacgen has accumulated a lot of successful experience in protein interaction analysis, and has assisted many customers around the world to complete projects with high efficiency and high quality. Our highly competitive prices and unprecedented expertise have earned us widespread acclaim. Request more information to find out how Profacgen can help.
References
Fill out this form and one of our experts will respond to you within one business day.