Incorporation of Unnatural Amino Acids
Subclass
Background
Introduction
Unnatural amino acids (UAAs), engineered to augment the genetic code, empower proteins with novel functionalities like bioorthogonal reactivity, enhanced stability, and site-specific modifications. Emerging from early 2000s breakthroughs in genetic code expansion, UAA technology now drives innovations in antibody-drug conjugates (ADCs), enzyme engineering, and precision therapeutics. By integrating orthogonal tRNA systems and codon reassignment, UAAs enable tailored solutions for drug development and sustainable biomanufacturing, positioning them as pivotal tools in synthetic biology and personalized medicine.
Profacgen: Your Partner in Advanced UAA Solutions
Profacgen excels in UAA-based protein engineering, offering streamlined services from design to validation. With cost-effective workflows, rapid turnaround, and 24/7 support, Profacgen ensures scalable solutions for academia and industry.
Unlock your protein's potential-partner with Profacgen Now!
Strategies for UAA Incorporation
1. Metabolic Incorporation: Simplified UAA Integration Through Native Pathways
Metabolic incorporation replaces natural amino acids (e.g., methionine) with structural analogs like azidohomoalanine (AHA) by hijacking cellular biosynthesis pathways. Engineered auxotrophic hosts uptake supplemented UAAs, enabling seamless integration during protein synthesis without genetic code expansion. This approach prioritizes simplicity and scalability, ideal for large-scale production of labeled proteins or biomaterials. AHA's bioorthogonal azide group supports click chemistry for applications such as protein tracking or ADC development. Though limited to analogs resembling natural residues, metabolic engineering optimizes yield and specificity, making it cost-effective for industrial biocatalysis and therapeutic protein modification.
2. Site-Specific Incorporation: Precision Engineering via Orthogonal Systems
Site-specific UAA insertion uses engineered tRNA/aminoacyl-tRNA synthetase (aaRS) pairs and repurposed nonsense codons (e.g., amber STOP) to achieve residue-level control. Orthogonal systems, often derived from microbial sources, exclusively charge UAAs like p-azido-L-phenylalanine (pAzF) for targeted integration. This enables precise functionalization—such as photo-crosslinking or bioconjugation—at predefined sites, critical for developing ADCs, FRET biosensors, or stabilized therapeutics. Combining codon optimization, suppressor tRNA enhancements, and genome editing, platforms like Profacgen's ensure high fidelity, advancing synthetic biology and precision medicine.
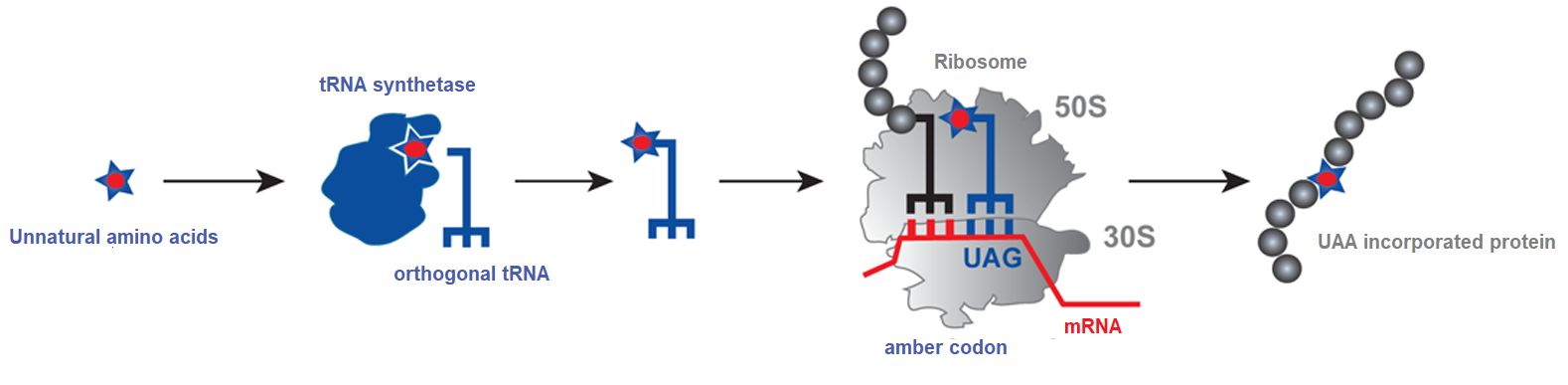 Fig1. UAA incorporation utilizes tRNA/aaRS pair in response to an amber codon.
Fig1. UAA incorporation utilizes tRNA/aaRS pair in response to an amber codon.
Applications of Unnatural Amino Acids
| Enhancing Protein Functionality |
|
| Bioorthogonal Chemistry |
|
| Drug Discovery and Development |
|
| Synthetic Biology |
|
Service Procedure
Sample Submission Requirements
To ensure the success of your UAA incorporation project, please follow these submission guidelines:
- Protein Sequence Information
- Provide the full amino acid sequence of the target protein and specify the exact site(s) for UAA incorporation.
- Indicate if codon optimization is needed for the expression system (e.g., E. coli, yeast, mammalian cells).
- UAA Specification
- Specify the type of unnatural amino acid required (e.g., p-azido-L-phenylalanine, homopropargylglycine).
- Indicate the desired bioorthogonal handle (e.g., azide, alkyne, ketone).
- Expression System
- Specify the preferred expression system (e.g., E. coli, yeast, mammalian cells).
- Note any specific vector requirements (e.g., plasmid backbone, promoter).
- Additional Information
- Specify if any affinity tags (e.g., His-tag, GST-tag) are required.
- Indicate purification requirements (e.g., Ni-NTA affinity chromatography).
Our UAA Incorporation Services
Experts at Profacgen provide the most up-to-date protein engineering technologies for incorporation of UAAs bearing bioorthogonal handles such as:
- Azides for click reaction or Staudinger ligation;
- Alkynes for click reaction;
- Ketones for amine-based reactions;
- Aryl halides for Palladium-catalyzed cross-coupling;
- Alkenes for metathesis.
Profacgen offers comprehensive services for incorporating unnatural amino acids (UAAs) into proteins, tailored to meet your specific needs:
| Customized Research Plans | UAA Synthesis and Modification | Protein Expression and Purification | Characterization and Analysis | Incorporation Strategies | Application-Specific Solutions |
|
|
|
|
|
|
Why Choose Profacgen?
- Expertise and Experience: Over a decade of experience in protein engineering with a team of leading experts.
- Advanced Technologies: State-of-the-art platforms for UAA synthesis, protein expression, and characterization.
- Quality and Reliability: ISO-certified processes with stringent quality control for high success rates and reproducibility.
- Customer Support: 24/7 customer service and technical support to assist you throughout your project.
Case Study
* NOTE: We prioritize confidentiality in our services to safeguard technology and intellectual property for enhanced future value and protection. The following case study has been shared with the client's consent.
Goal
The project involves pilot protein expression in an E. coli system with the incorporation of an unnatural amino acid (UAA). The specific amino acid site in the protein sequence is replaced with an amber codon (UAG), and a modified tRNA synthetase/tRNA pair is used to incorporate the UAA in vivo. The process starts with gene synthesis, codon optimization, and vector construction, followed by protein expression and purification. The primary goal is to express a protein in the E. coli system with the incorporation of an unnatural amino acid (UAA).
Results
The project successfully utilized a tRNA/aaRS pair to incorporate the UAA in response to an amber codon. The engineered aminoacyl-tRNA synthetase (aaRS) specifically recognized its cognate tRNA without interfering with endogenous tRNAs. The full-length protein containing the UAA was isolated and detected using a C-terminal His tag. The protein mutant (carrying an amber codon at a specific site upstream of the C-terminal His tag) was successfully expressed in the presence of the engineered aaRS.
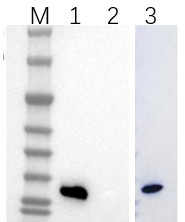 Fig2. Protein expression validation.
Fig2. Protein expression validation.
Lane M: Protein Marker; Lane 1: Wild protein
Lane 2: Protein mutant (contain amber codon at a specific site)
Lane 3: Protein mutant and engineered aaRS co expression.
Conclusions and Discussions
The project successfully synthesized a recombinant protein with a pAzF mutant gene and cloned it with a C-terminal His tag. The recombinant plasmid was co-expressed with the engineered aaRS in E. coli cells, and protein expression was successfully achieved.
Customer Testimonials
"Integrating unnatural amino acids via ProfacGen's platform revolutionized our antibody-drug conjugate (ADC) development. The site-specific incorporation improved payload precision in monoclonal antibodies, accelerating preclinical timelines. Their expertise in bioorthogonal chemistry saved us 3 months of method optimization. A must-try for targeted cancer therapy R&D teams!"
Dr. Emily Carter, Senior Research Lead | Biopharmaceutical Firm
"Exceptional PURE cell-free system support for engineered ribosomes! We achieved 92% incorporation efficiency of nitrotyrosine in artificial extracellular matrix proteins. Perfect balance between cost-effective solutions and high-yield expression systems. Critical for our tissue engineering projects requiring non-canonical amino acids."
Hiroshi Tanaka, CTO | Synthetic Biology Startup
"Game-changing tRNA synthetase engineering services for plant-based protein modification. Enabled stable pyrrolysine integration in drought-resistant crop enzymes. Their metabolic pathway optimization protocol outperformed two competitors we tested. Essential for sustainable agriculture innovation."
Dr. Priya Varma, Lab Director | Agricultural Biotech Co.
"Unmatched in amber stop codon suppression for viral vector vaccines. Achieved first-pass success with photocaged lysine in spike proteins for controlled immune activation. Their GMP-compatible platforms align perfectly with our mRNA-LNP formulation needs. Vital for next-gen vaccine platforms."
Michael O'Connor, Principal Scientist | Vaccine Developer
"Transformed our diagnostic assay development with clickable azidohomoalanine tags. Enabled rapid HRP/AP enzyme conjugation without compromising epitope binding. Their technical team's guidance on orthogonal translation systems was invaluable. Ideal for creating IVD kits requiring chemical biology precision."
Sofia Russo, Head of Proteomics | Diagnostic Tools Corp.
"Scaled up selenomethionine-incorporated industrial enzymes to 500L bioreactors using their modified CHO cell lines. Achieved 85% activity retention post-PEGylation – crucial for our detergent enzyme formulations. Their tech transfer documentation sets new industry benchmarks."
Dr. Liam Ng, Biomanufacturing Director | Industrial Enzymes Co.
FAQs
Resources
| Bacterial Expression | Fusion Protein Expression | Stable Cell Line Construction for Protein Expression | Protein Interaction Analysis Services | Screening and Profiling Services | Protein Labeling |
References:
- Niu W, Guo J. Cellular Site-Specific Incorporation of Noncanonical Amino Acids in Synthetic Biology. Chem Rev. 2024;124(18):10577-10617.
- Singh-Blom A.; et al. Residue-specific incorporation of unnatural amino acids into proteins in vitro and in vivo. Methods Mol Biol. 2013;978:93-114.
- Cox VE, Gaucher EA. Molecular Evolution Directs Protein Translation Using Unnatural Amino Acids. Curr Protoc Chem Biol. 2015;7(4):223-228.