We use cookies to understand how you use our site and to improve the overall user experience. This includes personalizing content and advertising. Read our Privacy Policy
GPCR Screening Service
Background
Introduction
Profacgen delivers cutting-edge high-throughput GPCR screening services that revolutionize GPCR-targeted drug discovery in pharmaceutical development. As G protein-coupled receptors (GPCRs) regulate critical cell signaling pathways and represent over 30% of current therapeutic targets, our optimized screening platform combines fluorescence-based assays, calcium flux analysis, and β-arrestin recruitment detection to identify promising lead compounds. Our customized screening packages feature rapid turnaround times (2-4 weeks), comprehensive data visualization, and mechanism-of-action validation, specifically designed for pharma companies and biotech startups pursuing first-in-class therapeutics.
Start Exploring Your Cost-effective Solutions Now!
What are GPCRs and Why They Matter
G protein-coupled receptors (GPCRs), characterized by their seven transmembrane helix architecture, are exclusive to eukaryotic organisms and serve as pivotal mediators of cellular communication. These membrane-bound proteins detect extracellular signals—including light-sensitive compounds, odors, hormones, neurotransmitters, and pheromones—and activate intracellular signaling cascades via G proteins or β-arrestin pathways. Functioning as molecular switches, GPCRs regulate processes ranging from immune responses and sensory perception to cell proliferation and cancer progression. Remarkably, nearly 40% of FDA-approved therapeutics target GPCRs, with 20% of global top-selling drugs—such as antihistamines, beta-blockers, and antipsychotics—leveraging their unique signaling mechanisms. This underscores their dominance in precision medicine and drug development.
The therapeutic potential of GPCRs stems from their structural diversity and functional versatility in GPCR signal transduction. Advances in GPCR drug discovery now focus on biased signaling modulation, allosteric site targeting, and orphan receptor deorphanization to address unmet needs in oncology, metabolic disorders, and neurological diseases. For instance, CRISPR-engineered cell models and cryo-EM structural analysis enable precise mapping of ligand-binding pockets, accelerating the design of subtype-selective agonists/antagonists. Pharmaceutical innovators increasingly prioritize GPCR targeting strategies to develop safer, more efficacious therapies, particularly in immuno-oncology and personalized medicine. As the $180 billion GPCR market expands, integrating AI-driven virtual screening and high-throughput assays remains critical for identifying next-generation drug candidates with minimized off-target effects. Explore cutting-edge GPCR research tools and screening platforms to unlock novel therapeutic avenues today.
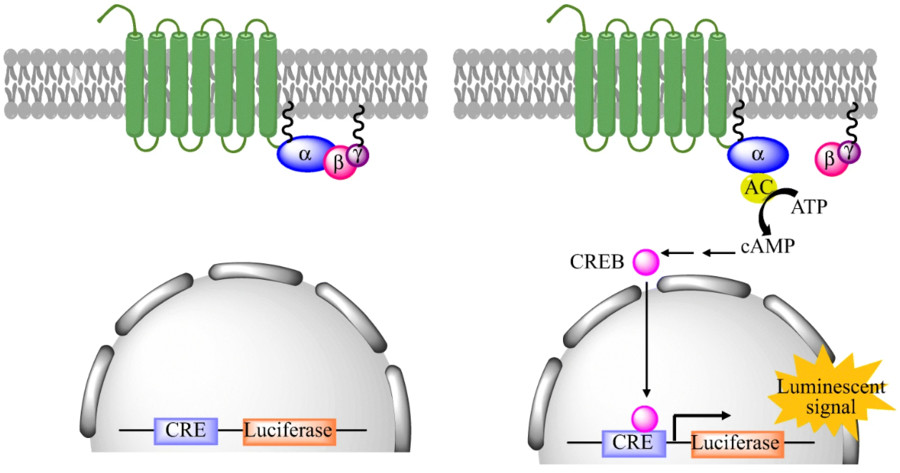 Fig2. Principle of GPCR activation induced reporter gene expression. (Kankanamge, et al., 2019)
Fig2. Principle of GPCR activation induced reporter gene expression. (Kankanamge, et al., 2019)
Applications of GPCR Screening
GPCR in Drug Development
High-throughput GPCR screening accelerates pharmaceutical innovation by streamlining target validation and lead compound optimization. Advanced platforms employ fluorescence-based assays and β-arrestin recruitment tests to analyze structure-activity relationships (SAR), enabling rapid identification of biased signaling modulators.
GPCR in Cancer Therapy
Screening identifies chemokine receptor antagonists to overcome immunotherapy resistance in solid tumors. For example, CXCR4/CXCL12 axis inhibitors block metastasis in breast cancer, while orphan GPCRs like GPR87 are validated as biomarkers for personalized oncology therapies. Our platforms now map tumor microenvironment interactions, supporting next-generation checkpoint inhibitors.
GPCR in Infectious Diseases
Viral entry inhibition strategies rely on GPCR screening to block host receptors like CCR5 (HIV) and ACE2 (SARS-CoV-2). Malaria research utilizes protease-activated receptor (PAR1) antagonists to disrupt parasite lifecycle, while bacterial quorum-sensing modulators target histidine kinase-coupled receptors.
Service Procedure
Sample Submission Requirements
To get started with our GPCR screening service, please follow these simple guidelines to prepare and submit your samples:
1. Sample Type
- Small Molecules: Provide compounds in dry form or dissolved in DMSO at 10 mM.
- Cell-Based Assays: Submit fresh or frozen cell pellets (≥1 × 10^6 cells) or cell lysates (≥1 mg/mL protein concentration).
- Proteins: Purified proteins with ≥95% purity and ≥1 µM concentration.
2. Quantity
- Compounds: Minimum of 1 mg dry or 100 µL of 10 mM DMSO solution.
- Cells: At least 1×106 cells per pellet.
- Proteins: Minimum of 1 µM concentration.
3. Packaging and Shipment
- Packaging: Use secure containers (e.g., snap-lock tubes for cells, vials for compounds).
- Shipping: Ship overnight on dry ice to maintain stability.
- Documentation: Include sample details (name, concentration, biological activity) and your contact information.
4. Storage
- Compounds: Store at -20°C.
- Cells/Proteins: Ship and store frozen on dry ice.
If you have any questions, feel free to reach out. We're here to help!
Our GPCR Screening Services
| Cell Membrane Preparation Our membrane preparation service delivers bioactive GPCR-rich cell membranes using HEK293 or baculovirus expression systems, optimized for radioligand binding and GTPγS assays. With >95% receptor functionality retention and batch-to-batch consistency, these membranes enable reliable screening for orphan receptor deorphanization and allosteric modulator identification in cardiovascular drug discovery. |
| GPCR Signal Detection We deploy multiplexed detection for cAMP, calcium flux, IP1/IP3, and β-arrestin recruitment, covering Gi/Gs/Gq-coupled receptors. Customized reporter gene assays validate pathway-specific activation, critical for characterizing biased signaling in cancer immunotherapy and metabolic disorder targets like GLP-1R. Other signal detection assays: Ligand-receptor binding assay (using radio-labeled isotope ligand): arrestin recruitment, receptor internalization Cell based high content screening (HCS) |
| High-Throughput Screening Our automated platform screens 100,000+ compounds/day via 1,536-well formats, integrating virtual screening libraries and AI-driven hit prioritization. This accelerates SAR optimization for CNS drug candidates and antiviral leads, reducing lead discovery timelines by 40% compared to conventional methods. |
| Other Services We can also help with design your customized GPCR analytical scheme based on your requirements and your preliminary results, such as GPCR agonist screening, GPCR antagonist screening, GPCR allosteric modulator screening, GPCR inverse agonist screening, etc. |
Our Technology Platforms
Scalable imaging, detection instruments and liquid handling systems
Optimized cell-based assay platforms

Ready-to-use GPCR cell lines
Why Choose Profacgen?
Technical Expertise in Proximity-Dependent Labeling
Expert Consultation: Dedicated pharmacologists provide end-to-end support—assay design, mechanism-of-action studies, and clinical candidate selection-for complex targets like GPCR heterodimers.
Cutting-Edge Technologies: Proprietary platforms and optogenetics-enabled GPCR activation address undruggable targets in fibrosis and rare diseases.
Cost-Effective Scaling: Flexible pricing models reduce screening costs by 30% for academic labs and startups through shared compound libraries or pooled receptor panels.
IP Protection: Secure, encrypted data management and NDAs safeguard client innovations in competitive areas like allosteric modulator discovery.
Global Accessibility: Regional hubs (US, EU) offer 24/7 project tracking and same-day shipping of critical reagents (fluorescent probes, cryopreserved cells).
Case Study
* NOTE: We prioritize confidentiality in our services to safeguard technology and intellectual property for enhanced future value and protection. The following case study has been shared with the client's consent.
Goal
The service quotation is for conducting functional assays for a GPCR target in an agonist mode, based on the company's GPCR platform. The customer provides one compound for testing. The goal is to perform the GPCR functional assay to assess the interaction between the customer's compound and the GPCR target.
Results
As the customer's requirements, GPCR target was used in cell-based assay with the start concentration at 1000 μM followed a 10x dilution.
Table 1 Testing requirements
| Compound name | Assay name | Top testing concentration | Dilution factor |
| Test compound | Human AM2 (CLR/RAMP3) Calcitonin GPCR Cell Based Agonist Arrestin Assay | 1000 μM | 10X |
| Test compound | Human CB1 Cannabinoid GPCR Cell Based Agonist cAMP Assay | 1000 μM | 10X |
The compound activity assays were completed using the GPCR platform. The detailed results are shown in Table 2 of the report. The key findings are as follows:
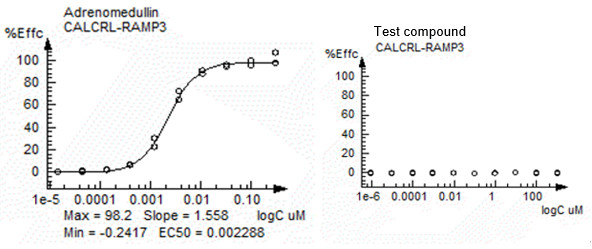 Fig2. AM2 (CLR-RAMP3) assay result with agonist mode based on arrestin pathway.
Fig2. AM2 (CLR-RAMP3) assay result with agonist mode based on arrestin pathway.
Left: Adrenomedullin (agonist control); Right: customer compound
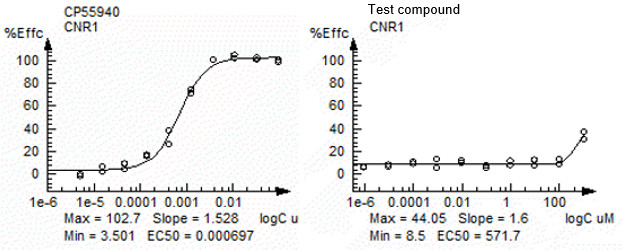 Fig3. CB1 (CNR1) assay result with agonist mode based on cAMP pathway.
Fig3. CB1 (CNR1) assay result with agonist mode based on cAMP pathway.
Left: CP55940 (agonist control); Right: Test compound
Table 2 Compound activity with the selected GPCR Assays
| Compound Name | Assay Name | Assay Target | Result Type | EC50 (µM) | Hill | Curve Bottom | Curve Top | Max Response |
| Adrenomedullin | Arrestin | AM2 (CALCRL-RAMP3) | EC50 | 0.002288337 | 1.5577 | -0.24175 | 98.198 | 101.68 |
| CP55940 | cAMP | CB1 (CNR1) | EC50 | 0.000696963 | 1.5281 | 3.5012 | 102.71 | 101.02 |
| Test sample | Arrestin | AM2 (CALCRL-RAMP3) | EC50 | >1000 | - | - | - | 0 |
| Test sample | cAMP | CB1 (CNR1) | EC50 | 571.7155 | 1.6 | 8.5002 | 44.05 | 33.735 |
Customer Testimonials
"Flexible allosteric modulator screening packages enabled rapid prioritization of three GPCR targets without infrastructure investment. Their FDA-aligned validation protocols were pivotal in securing co-development partnerships with top-tier pharma."
Dr. Sophia L., Global BD Director (Rare Disease Solutions, Multinational Biotech)
"Profacgen's GPCR screening services streamlined our CXCR4 antagonist development for solid tumors. Their high-throughput calcium flux assays identified clinical candidates in 5 weeks, aligning with our Phase II trial timelines. The scalability for GMP-grade validation was exceptional."
Dr. Emily S., R&D Director (Global Oncology Pharma)
"Integrating their β-arrestin recruitment assays into our CNS drug pipeline resolved target engagement uncertainties. The customized GPCR panels for orphan receptors accelerated IND-enabling studies by 8 months, exceeding internal benchmarks."
Dr. Raj Patel, VP of Drug Discovery (Biotech Firm)
"By outsourcing GLP-1R agonist screening, we reduced operational costs by 35% while maintaining FDA-compliant data rigor. Their multiplexed cAMP/IP1 detection supported seamless transition from hit identification to preclinical tox studies."
Ms. Hiroko T., Senior Project Lead (Metabolic Disorders Division)
"Leveraging their GPCR dimerization HCS platform, we decoded resistance mechanisms in checkpoint inhibitor therapies for pharma clients. The high-content screening datasets directly informed two ongoing Phase I/II collaborations."
Dr. Lars V., Head of Translational Research (Immuno-Oncology CRO)
"Profacgen's CCR5 antagonist screening for HIV delivered industry-standard IC50 curves and mechanistic insights. Their radioligand binding assays provided the reproducibility our partners demand for global licensing agreements."
Mr. Carlos M., Chief Scientific Officer (Infectious Disease Therapeutics)
"Flexible allosteric modulator screening packages enabled rapid prioritization of three GPCR targets without infrastructure investment. Their FDA-aligned validation protocols were pivotal in securing co-development partnerships with top-tier pharma."
Dr. Sophia L., Global BD Director (Rare Disease Solutions, Multinational Biotech)
"Profacgen's GPCR screening services streamlined our CXCR4 antagonist development for solid tumors. Their high-throughput calcium flux assays identified clinical candidates in 5 weeks, aligning with our Phase II trial timelines. The scalability for GMP-grade validation was exceptional."
Dr. Emily S., R&D Director (Global Oncology Pharma)
FAQs
Resources
| Transporter Screening Service | Ion Channel Screening Service | Aptamer Development Service | Receptor for Temperature and Touch Research and Discovery | Single Molecular Array Testing |
Reference:
- Kankanamge D.; et al. Optical approaches for single-cell and subcellular analysis of GPCR-G protein signaling. Anal Bioanal Chem. 2019;411(19):4481-4508.
